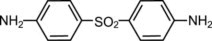Label: DAPSONE gel
- NDC Code(s): 0713-0886-18, 0713-0886-60
- Packager: Cosette Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DAPSONE GEL, 5% safely and effectively. See full prescribing information for DAPSONE GEL, 5%. DAPSONE gel, 5%, for topical use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Dapsone Gel, 5%, is indicated for the topical treatment of acne vulgaris.
-
2 DOSAGE AND ADMINISTRATION
For topical use only. Not for oral, ophthalmic, or intravaginal use. After the skin is gently washed and patted dry, apply approximately a pea-sized amount of Dapsone Gel, 5%, in a thin layer to ...
-
3 DOSAGE FORMS AND STRENGTHS
Gel, 5%. Each gram of Dapsone Gel contains 50 mg of dapsone in a white to pale yellow gel.
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Methemoglobinemia - Cases of methemoglobinemia, with resultant hospitalization, have been reported postmarketing in association with Dapsone Gel, 5% treatment ...
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
7.1 Trimethoprim-Sulfamethoxazole - A drug-drug interaction study evaluated the effect of the use of Dapsone Gel, 5%, in combination with double strength (160 mg/800 mg ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - There are no available data on Dapsone Gel, 5%, use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. In animal ...
-
11 DESCRIPTION
Dapsone Gel, 5%, contains dapsone, a sulfone, in an aqueous gel base for topical dermatologic use. Dapsone Gel, 5% is a gritty translucent material with visible drug substance particles ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism of action of dapsone gel in treating acne vulgaris is not known. 12.3 Pharmacokinetics - An open-label study compared the pharmacokinetics of dapsone ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Dapsone was not carcinogenic to rats when orally administered to females for 92 weeks or males for 100 weeks at dose levels up to 15 ...
-
14 CLINICAL STUDIES
Two randomized, double-blind, vehicle-controlled, clinical trials were conducted to evaluate Dapsone Gel, 5%, for the treatment of subject with acne vulgaris (N=1475 and 1525). The trials were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
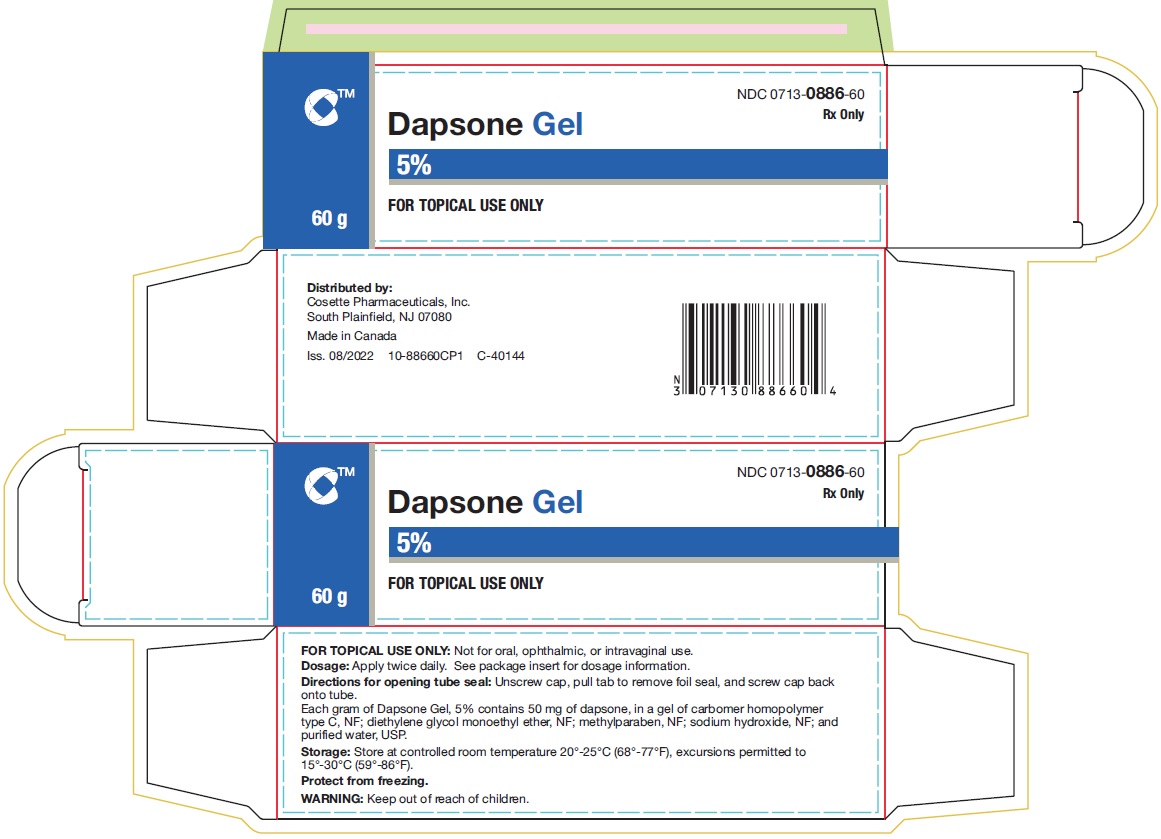
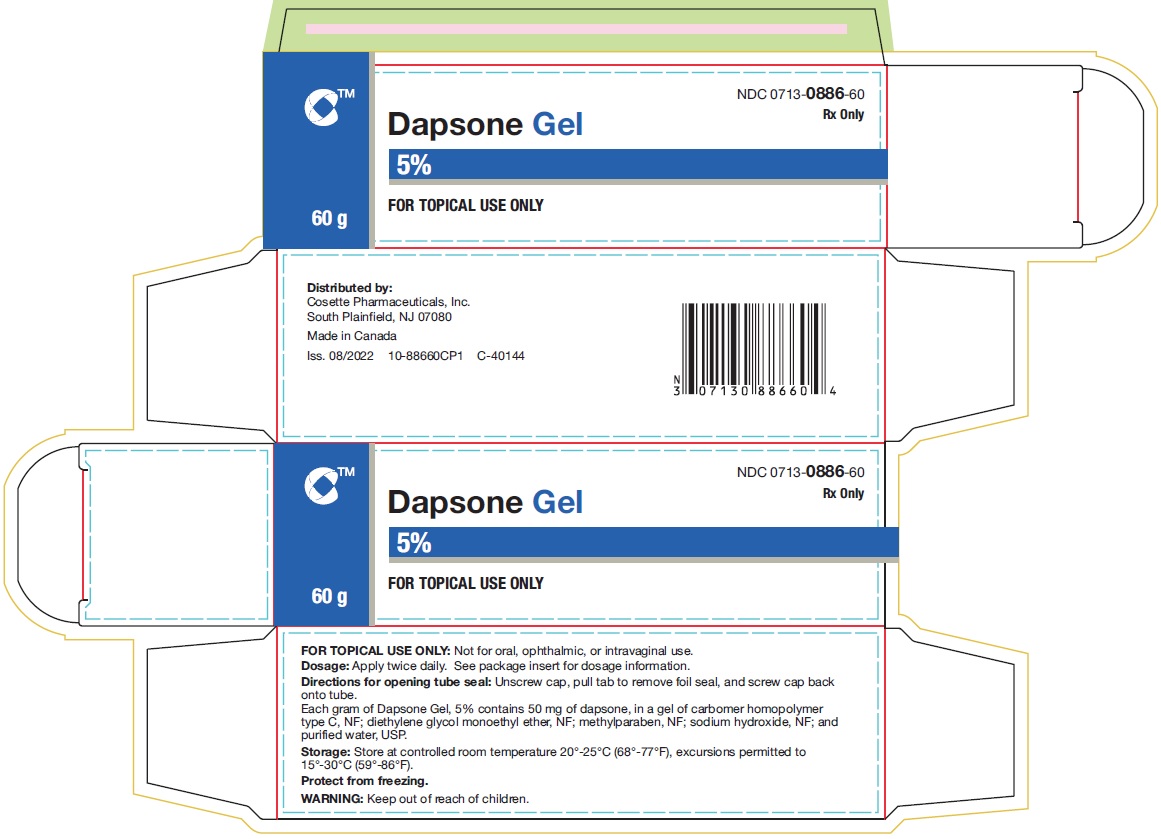
Dapsone Gel, 5%, is supplied in the following size tubes: NDC 0713-0886-60 - 60 gram laminate tube - NDC 0713-0886-18 - 90 gram laminate tube - Store ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Hematological Effects - • Inform patients that methemoglobinemia can occur with topical ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Rev. 08/2022 - PATIENT INFORMATION - Dapsone (DAP-zōn) Gel, 5% Important: For use on skin only ...
-
PRINCIPAL DISPLAY PANELNDC 0713-0886-60 - Rx Only - Dapsone Gel - 5% FOR TOPICAL USE ONLY - 60 g - Cosette Pharmaceuticals, Inc. NDC 0713-0886-18 - Rx Only - Dapsone Gel - 5% FOR TOPICAL USE ONLY - 90 ...
-
INGREDIENTS AND APPEARANCEProduct Information