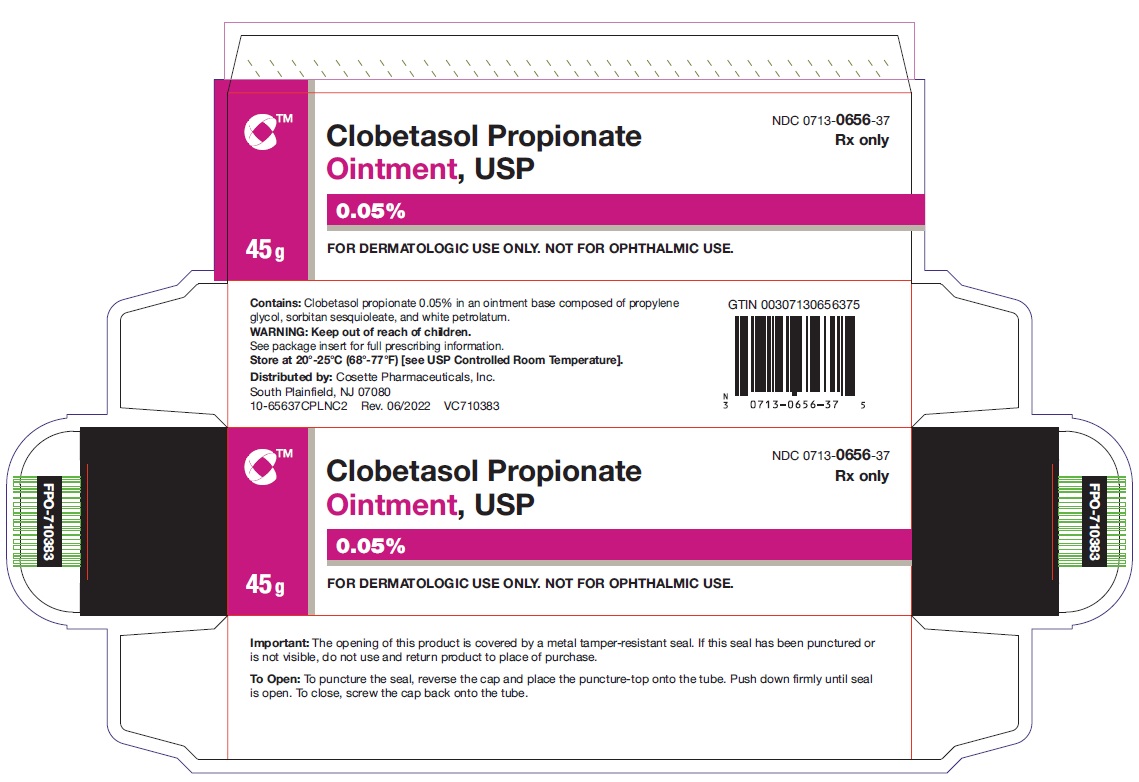
Label: CLOBETASOL PROPIONATE ointment
- NDC Code(s): 0713-0656-15, 0713-0656-31, 0713-0656-37, 0713-0656-60
- Packager: Cosette Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
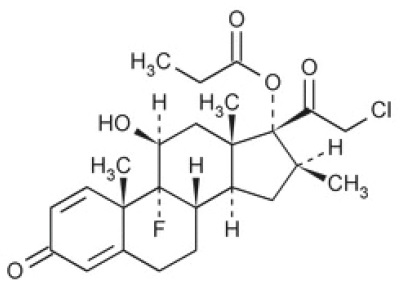
DESCRIPTIONClobetasol Propionate Ointment, USP 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a ...
-
CLINICAL PHARMACOLOGYLike other topical corticosteroids, clobetasol propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical ...
-
PharmacokineticsThe extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing with ...
-
INDICATIONS AND USAGEClobetasol Propionate Ointment is a super-high potency corticosteroid formulation indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses ...
-
CONTRAINDICATIONSClobetasol Propionate Ointment, 0.05% is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
- PRECAUTIONS
-
GeneralClobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at doses as low as 2 g per day. Systemic absorption of topical corticosteroids ...
-
Information for PatientsPatients using topical corticosteroids should receive the following information and instructions: 1. This medication is to be used as directed by the physician. It is for external use ...
-
Laboratory TestsThe following tests may be helpful in evaluating patients for HPA axis suppression: ACTH stimulation test A.M. plasma cortisol test Urinary ...
-
Carcinogenesis, Mutagenesis, Impairment of FertilityLong-term animal studies have not been performed to evaluate the carcinogenic potential of clobetasol propionate. Studies in the rat following subcutaneous administration at dosage levels ...
-
PregnancyTeratogenic Effects:Pregnancy Category C. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some ...
-
Nursing MothersSystemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known ...
-
Pediatric UseSafety and effectiveness of Clobetasol Propionate Ointment in pediatric patients have not been established. Use in children under 12 years of age is not recommended. Because of a higher ratio of ...
-
Geriatric UseClinical studies of clobetasol propionate drug products in US clinical trials did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from ...
-
ADVERSE REACTIONSIn controlled clinical trials, the most frequent adverse reactions reported for Clobetasol Propionate Ointment were burning sensation, irritation, and itching in 0.5% of treated patients. Less ...
-
OVERDOSAGETopically applied Clobetasol Propionate Ointment can be absorbed in sufficient amounts to produce systemic effects (see - PRECAUTIONS).
-
DOSAGE AND ADMINISTRATIONApply a thin layer of Clobetasol Propionate Ointment to the affected skin areas twice daily and rub in gently and completely (see - INDICATIONS AND USAGE). Clobetasol Propionate Ointment ...
-
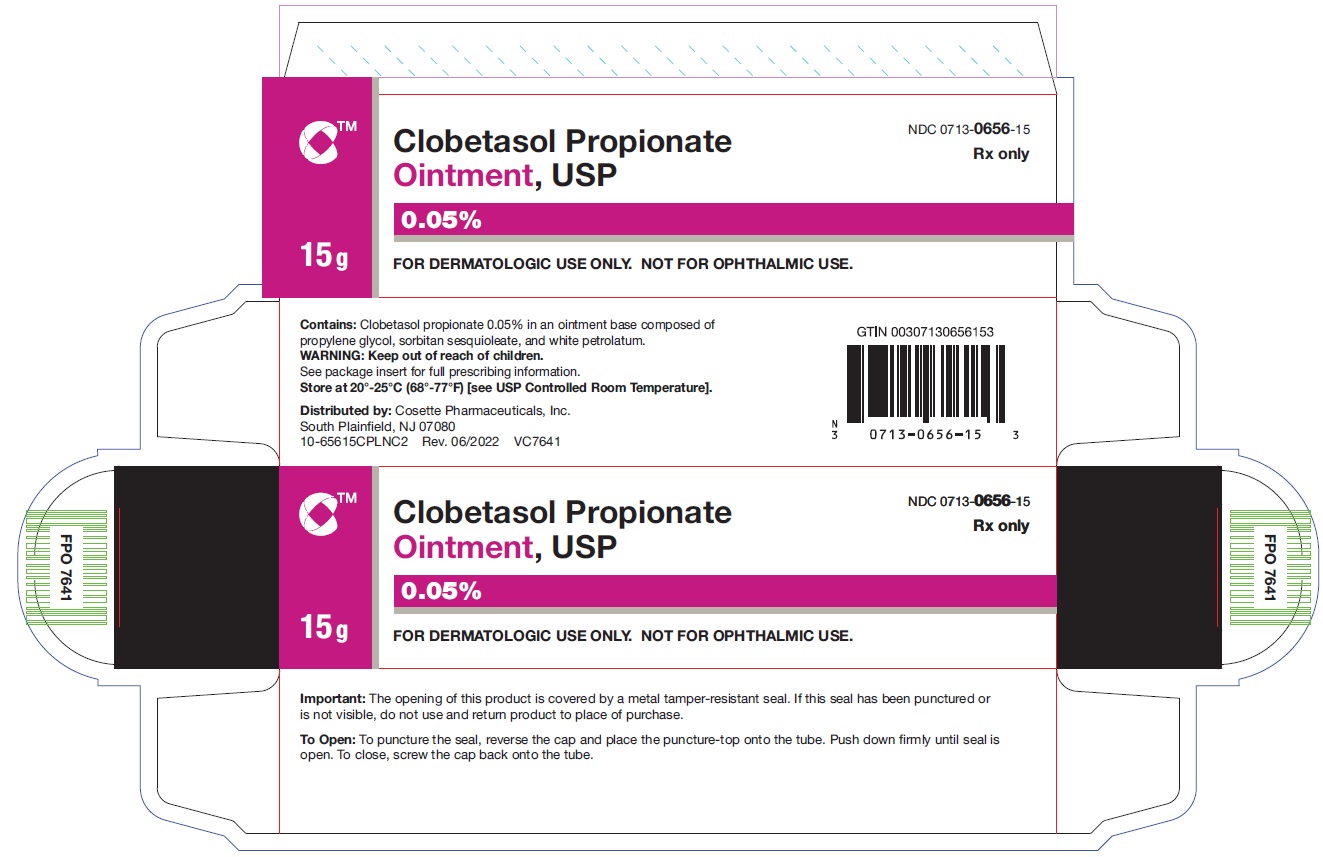
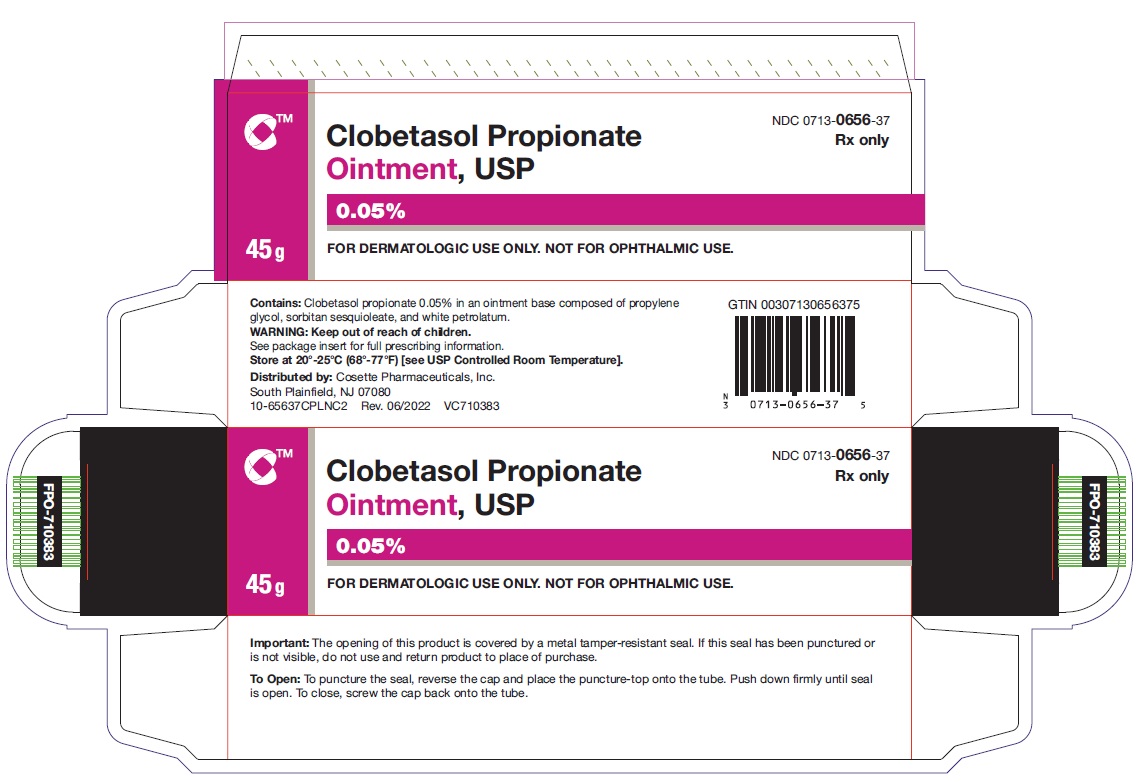
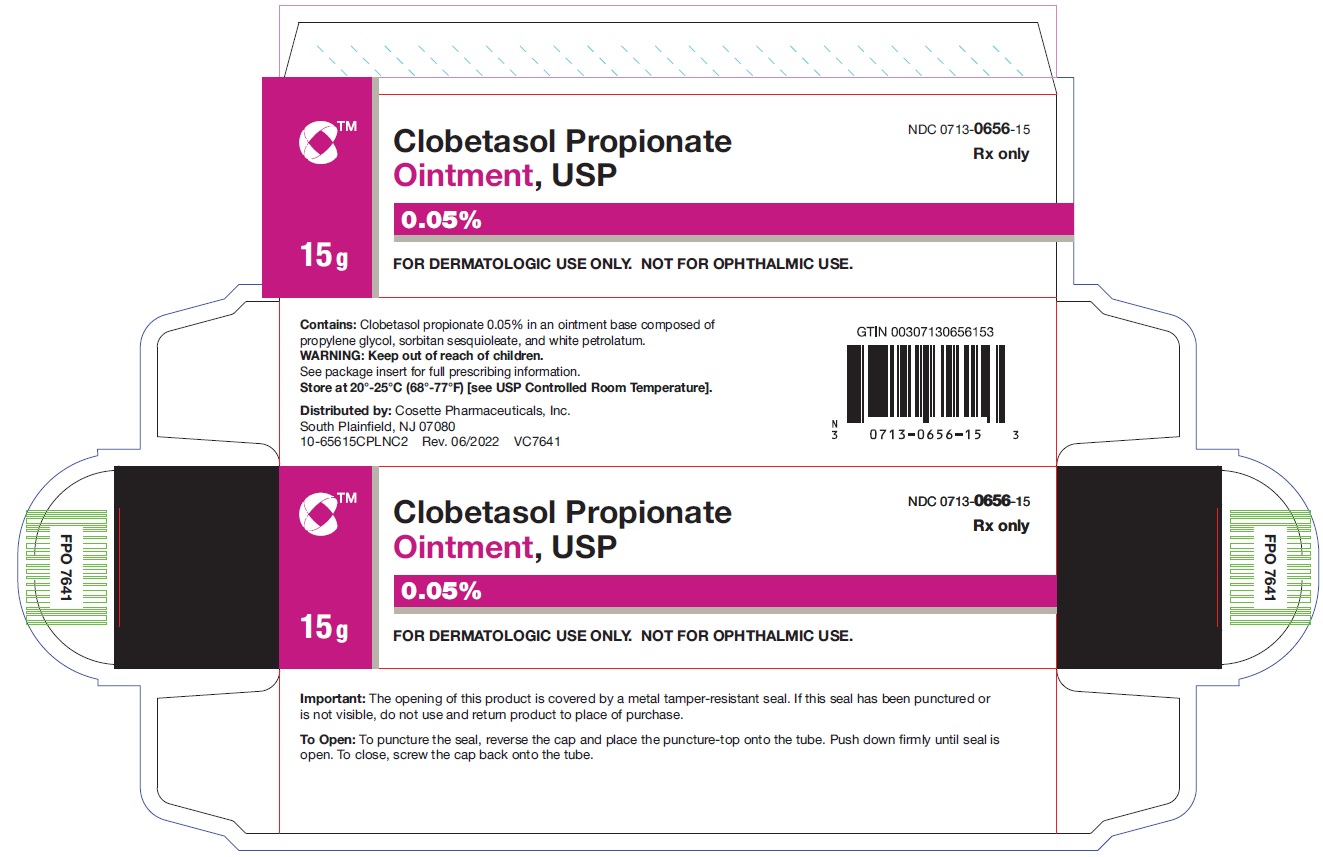
HOW SUPPLIEDClobetasol Propionate Ointment, USP 0.05% is supplied in: 15 g tubes (NDC 0713-0656-15) 30 g tubes (NDC 0713-0656-31) 45 g tubes (NDC 0713-0656-37) 60 g tubes (NDC ...
-
PRINCIPAL DISPLAY PANELNDC 0713-0656-15 - Clobetasol Propionate Ointment,USP 0.05% 15 g - Rx only - FOR DERMATOLOGIC USE ONLY - NOT FOR OPHTHALMIC USE - Cosette Pharmaceuticals, Inc. NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information