Label: MOMETASONE FUROATE cream
- NDC Code(s): 0713-0634-15, 0713-0634-37
- Packager: Cosette Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MOMETASONE FUROATE CREAM, USP safely and effectively. See full prescribing information for MOMETASONE FUROATE CREAM, USP ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMometasone furoate cream, USP, 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 2 years of age ...
-
2 DOSAGE AND ADMINISTRATIONApply a thin film of mometasone furoate cream, USP, 0.1% to the affected skin areas once daily. Mometasone furoate cream, USP, 0.1% may be used in pediatric patients 2 years of age or older. Since ...
-
3 DOSAGE FORMS AND STRENGTHSCream, 0.1%. Each gram of mometasone furoate cream, USP, 0.1% contains 1 mg of mometasone furoate in a white to off-white smooth and homogenous cream base.
-
4 CONTRAINDICATIONSMometasone furoate cream, USP, 0.1% is contraindicated in those patients with a history of hypersensitivity to any of the components in the preparation.
-
5 WARNINGS AND PRECAUTIONS5.1 Effects on Endocrine System - Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNo drug-drug interaction studies have been conducted with mometasone furoate cream, USP, 0.1%.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. Therefore, mometasone furoate cream, USP, 0.1% should be ...
-
10 OVERDOSAGETopically applied mometasone furoate cream, USP, 0.1% can be absorbed in sufficient amounts to produce systemic effects [ see Warnings and Precautions ( 5.1) ].
-
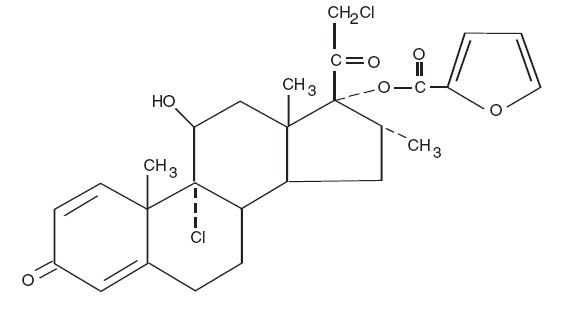
11 DESCRIPTIONMometasone furoate cream, USP, 0.1% contains mometasone furoate for topical use. Mometasone furoate is a synthetic corticosteroid with anti-inflammatory activity. Chemically, mometasone ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Like other topical corticosteroids, mometasone furoate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of mometasone furoate cream, USP, 0.1% ...
-
14 CLINICAL STUDIESThe safety and efficacy of the mometasone furoate cream, USP, 0.1% for the treatment of corticosteroid-responsive dermatoses were evaluated in two randomized, double-blind, vehicle-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMometasone furoate cream, USP, 0.1% is supplied in 15 g (NDC 0713-0634-15) and 45 g (NDC 0713-0634-37) tubes; boxes of one. Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling ( Patient Information). Inform patients of the following: • Use mometasone furoate cream, USP, 0.1% as directed by the ...
-
Patient InformationMometasone Furoate Cream, USP, 0.1% (Mo-meta-sone fur-o-ate) Important information: Mometasone Furoate Cream, USP, 0.1% is for use on skin only.Do not use mometasone furoate cream ...
-
PRINCIPAL DISPLAY PANEL
NDC 0713-0634-15 - Mometasone Furoate Cream, USP 0.1% 15 g - Rx only - FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE. Cosette Pharmaceuticals, Inc. NDC 0713-0634-37 - Mometasone ...
-
INGREDIENTS AND APPEARANCEProduct Information