Label: ANUCORT-HC- hydrocortisone acetate suppository
- NDC Code(s): 0713-0503-01, 0713-0503-12, 0713-0503-24
- Packager: Cosette Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
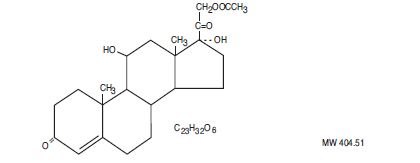
DESCRIPTIONHydrocortisone Acetate is a corticosteroid designated chemically as pregn-4-ene 3, 20-dione,21-(acetyloxy)-11, 17-dihydroxy-(11ß) with the following structural formula: Each rectal suppository ...
-
CLINICAL PHARMACOLOGYIn normal subjects, about 26% of hydrocortisone acetate is absorbed when the suppository is applied to the rectum. Absorption of hydrocortisone acetate may vary across abraded or inflamed ...
-
INDICATIONS AND USAGEHydrocortisone acetate suppositories are indicated for use in inflamed hemorrhoids, post-irradiation (factitial) proctitis; as an adjunct in the treatment of chronic ulcerative colitis; cryptitis ...
-
CONTRAINDICATIONSHydrocortisone acetate suppositories are contraindicated in those patients having a history of hypersensitivity to hydrocortisone acetate or any of the components.
-
PRECAUTIONSDo not use hydrocortisone acetate suppositories unless adequate proctologic examination is made. If irritation develops, the product should be discontinued and appropriate therapy ...
-
INFORMATION FOR PATIENTSStaining of fabric may occur with use of the suppository. Precautionary measures are recommended.
-
PREGNANCY CATEGORY CIn laboratory animals, topical steroids have been associated with an increase in the incidence of fetal abnormalities when gestating females have been exposed to rather low dosage levels. There ...
-
ADVERSE REACTIONSThe following local adverse reactions have been reported with hydrocortisone acetate suppositories: burning, itching, irritation, dryness, folliculitis, hypopigmentation, allergic contact ...
-
DRUG ABUSE AND DEPENDENCEDrug abuse and dependence have not been reported in patients treated with hydrocortisone acetate suppositories.
-
OVERDOSAGEIf signs and symptoms of systemic overdosage occur, discontinue use.
-
DOSAGE AND ADMINISTRATIONFor rectal administration. Detach one suppository from strip of suppositories. Hold suppository upright. Separate tabs at top opening and pull downward from the pointed end. Continue pulling ...
-
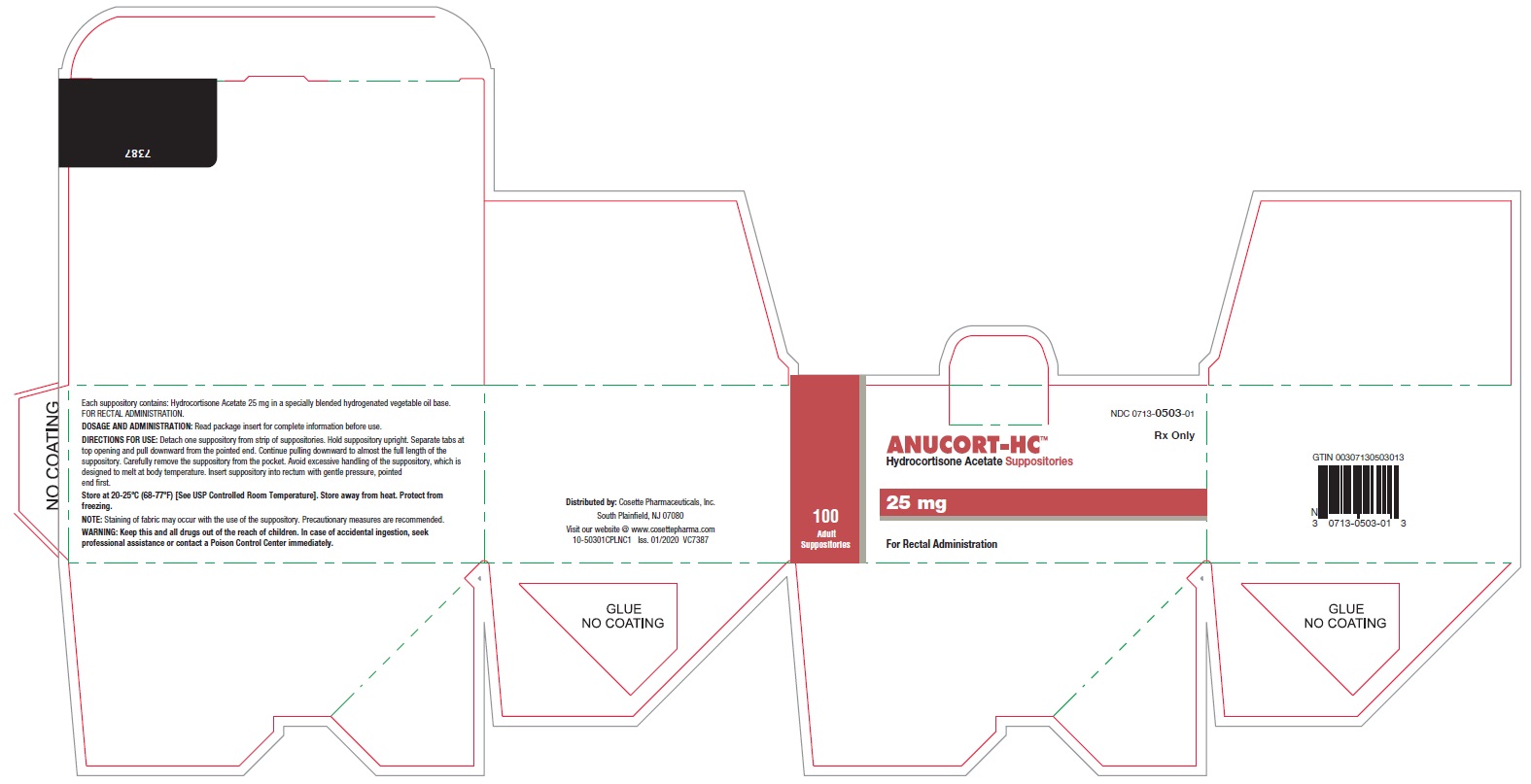
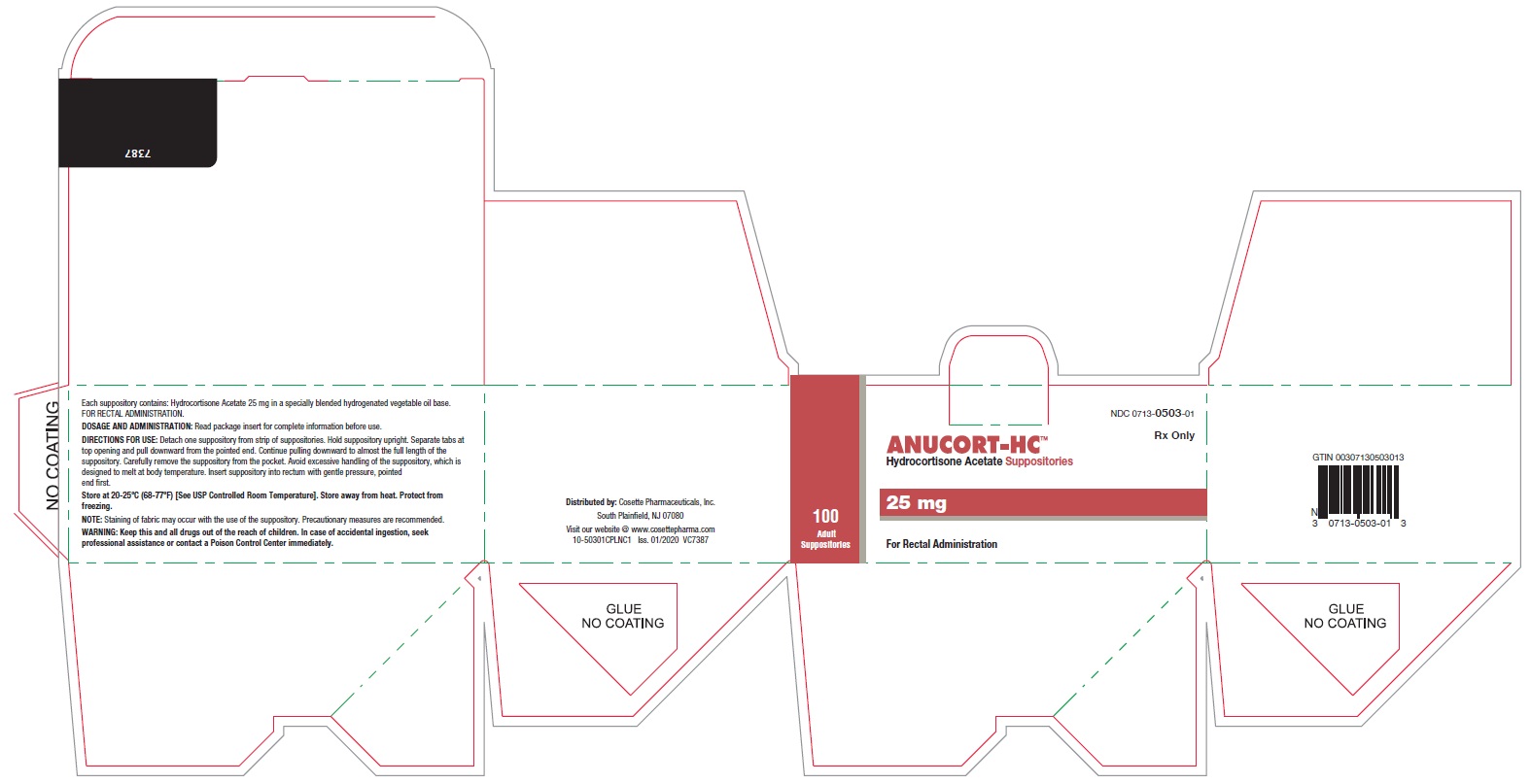
HOW SUPPLIEDBoxes of 12 suppositories NDC 0713-0503-12 - Boxes of 24 suppositories NDC 0713-0503-24 - Boxes of 100 suppositories NDC 0713-0503-01
-
STORAGE AND HANDLINGStore at 20-25°C (68°-77°F) [See USP Controlled Room Temperature]. Store away from heat. Protect From Freezing. Distributed by: Cosette Pharmaceuticals, Inc. South Plainfield, NJ ...
-
PRINCIPAL DISPLAY PANELNDC 0713-0503-12 - ANUCORT-HC - TM - Hydrocortisone Acetate Suppositories - 25 mg - Rx only - For Rectal Administration - 12 Adult Suppositories - Cosette Pharmaceuticals, Inc. NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information