Label: TRIAMCINOLONE ACETONIDE injection, suspension
- NDC Code(s): 0703-0241-01, 0703-0243-01, 0703-0245-01
- Packager: Teva Parenteral Medicines, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONNOT FOR USE IN NEONATES - CONTAINS BENZYL ALCOHOL - For Intramuscular or Intra-articular Use Only - NOT FOR INTRAVENOUS, INTRADERMAL, INTRAOCULAR, EPIDURAL, OR INTRATHECAL USE - Rx only
-
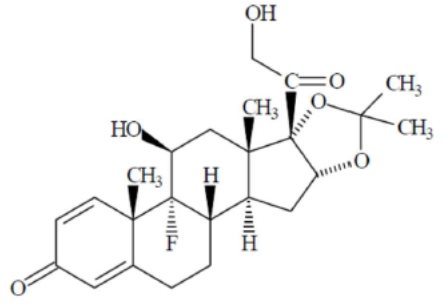
DESCRIPTIONTriamcinolone acetonide injectable suspension USP is a synthetic glucocorticoid corticosteroid with anti-inflammatory action. THIS FORMULATION IS SUITABLE FOR INTRAMUSCULAR AND INTRA-ARTICULAR USE ...
-
CLINICAL PHARMACOLOGYGlucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Naturally occurring glucocorticoids (hydrocortisone and ...
-
INDICATIONS AND USAGEIntramuscular - Where oral therapy is not feasible, injectable corticosteroid therapy, including triamcinolone acetonide injectable suspension is indicated for intramuscular use as ...
-
CONTRAINDICATIONSTriamcinolone acetonide injectable suspension is contraindicated in patients who are hypersensitive to any components of this product (see WARNINGS: General). Intramuscular corticosteroid ...
-
WARNINGSSerious Neurologic Adverse Reactions with Epidural Administration - Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids (see ...
-
PRECAUTIONSGeneral - This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial. The lowest ...
-
ADVERSE REACTIONS(listed alphabetically under each subsection) The following adverse reactions may be associated with corticosteroid therapy: Allergic reactions: Anaphylaxis including death ...
-
OVERDOSAGETreatment of acute overdosage is by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy, the dosage of the corticosteroid ...
-
DOSAGE AND ADMINISTRATIONGeneral - NOTE: CONTAINS BENZYL ALCOHOL (see PRECAUTIONS). The initial dose of triamcinolone acetonide injectable suspension may vary from 2.5 mg to 100 mg per day depending on the specific ...
-
HOW SUPPLIEDTriamcinolone acetonide injectable suspension USP is a sterile, isotonic, nonpyrogenic white parenteral suspension supplied in vials providing 40 mg triamcinolone acetonide per ...
-
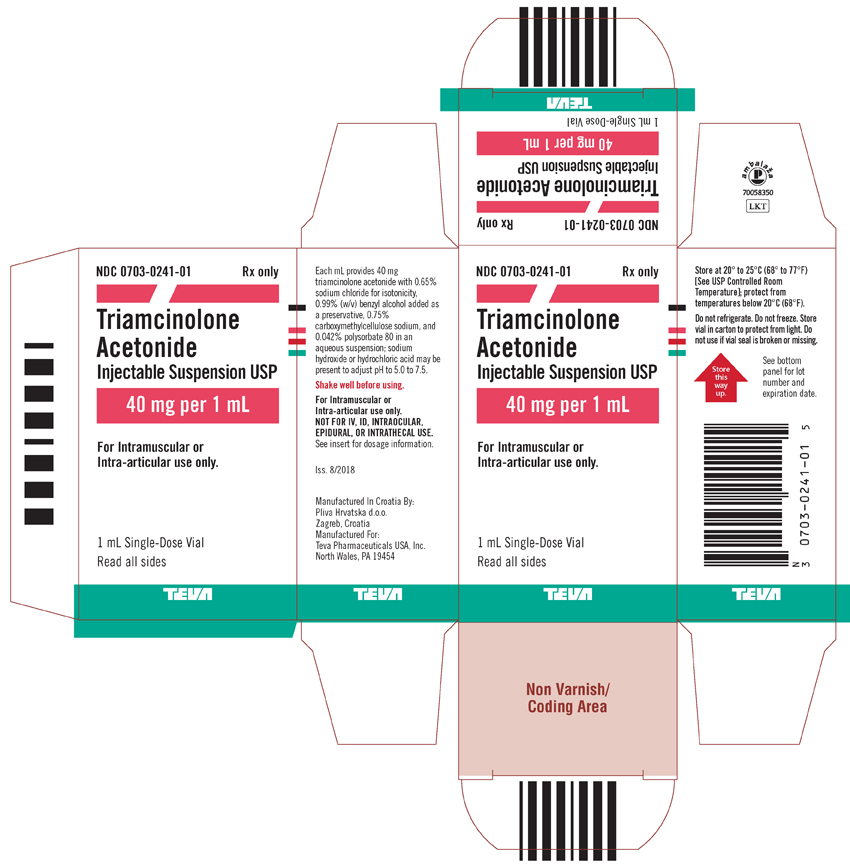
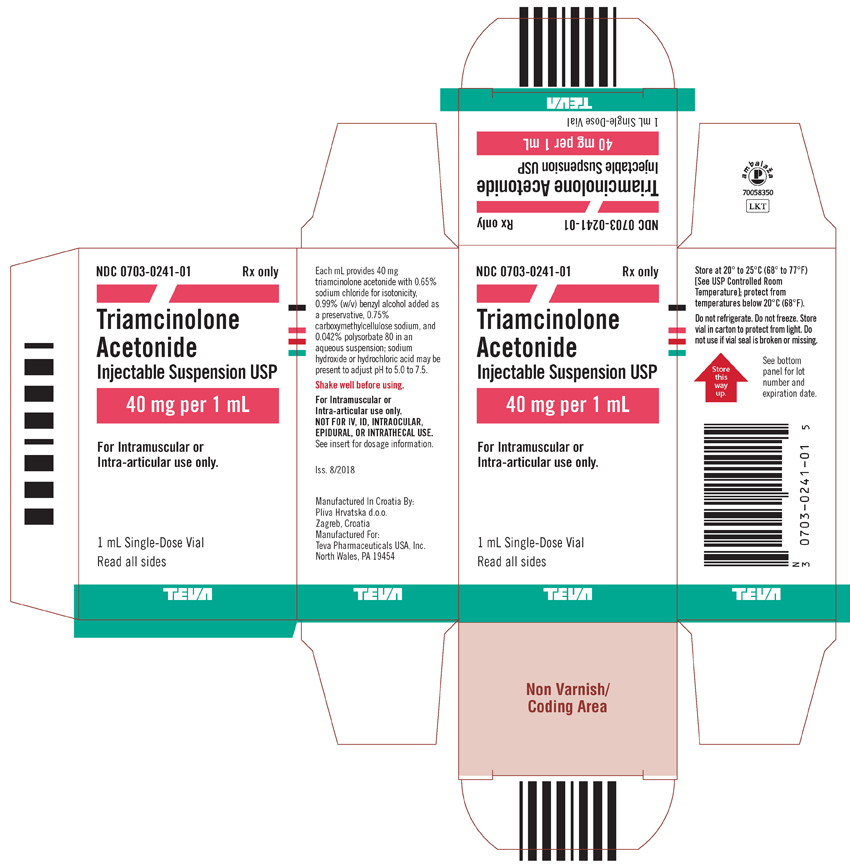
Package/Label Display PanelNDC 0703-0241-01 - Rx only - Triamcinolone Acetonide Injectable Suspension USP - 40 mg per 1 mL - For intramuscular or intra-articular use only. 1 mL Single-Dose Vial - Read all sides
-
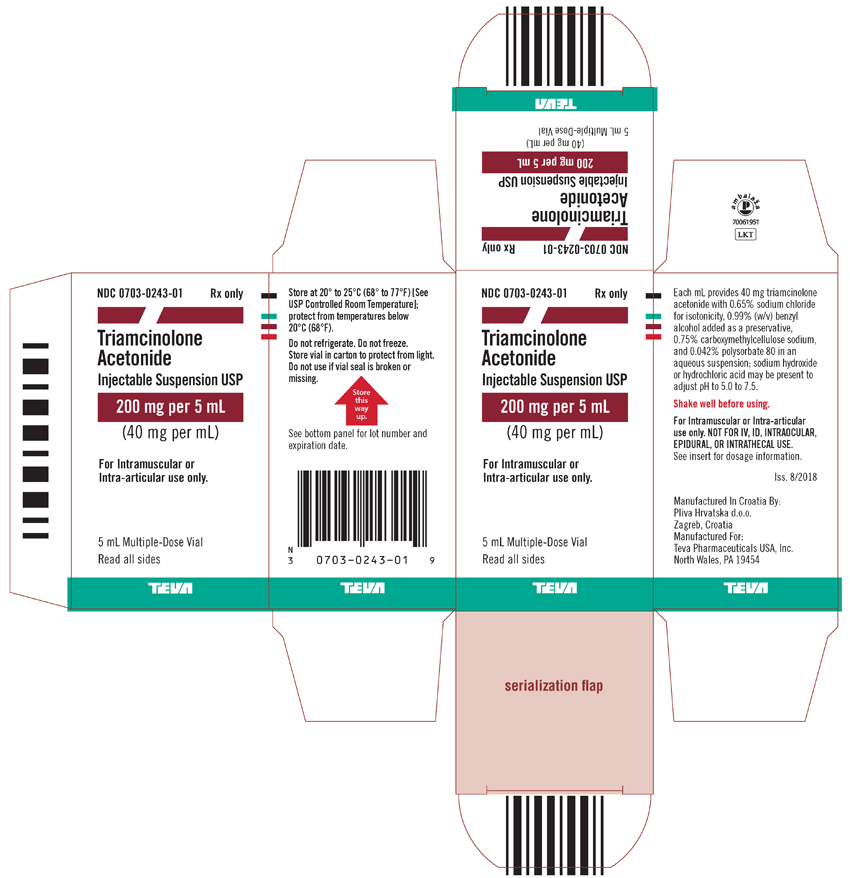
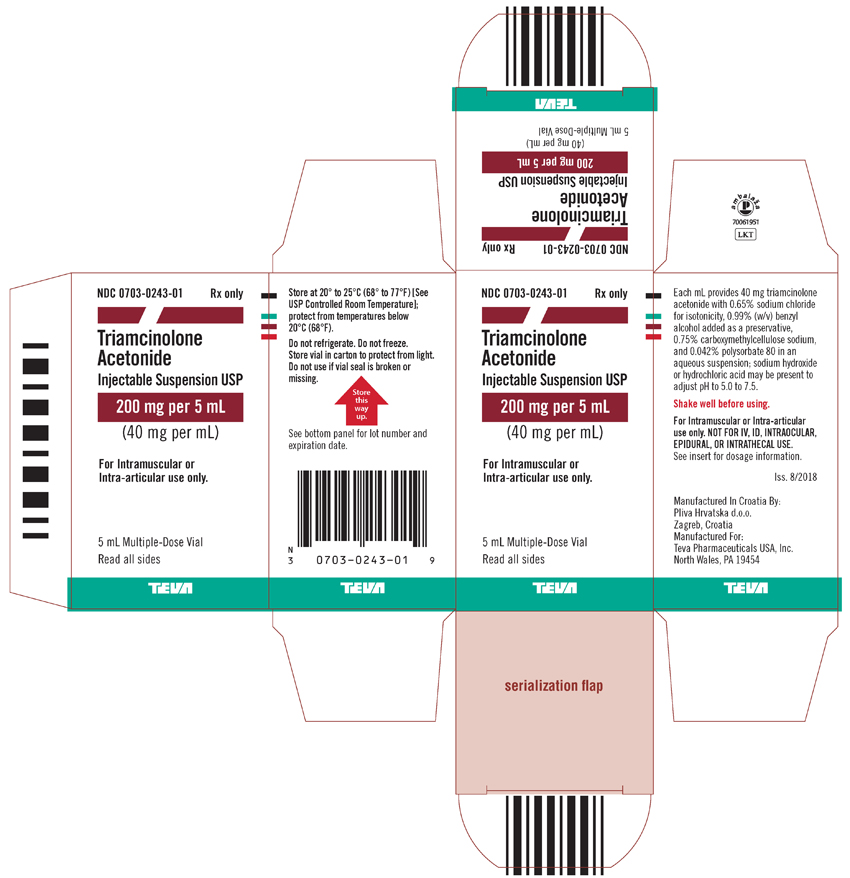
Package/Label Display PanelNDC 0703-0243-01 - Rx only - Triamcinolone Acetonide Injectable Suspension USP - 200 mg per 5 mL - (40 mg per 1 mL) For intramuscular or intra-articular use only. 5 mL Multiple-Dose Vial - Read all ...
-
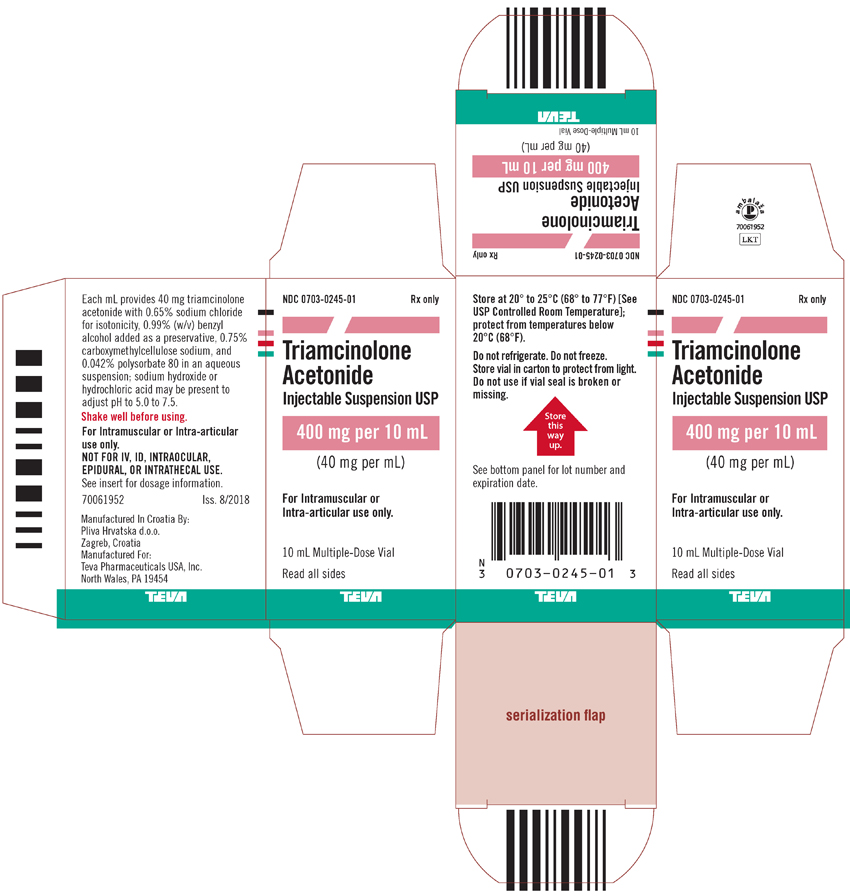
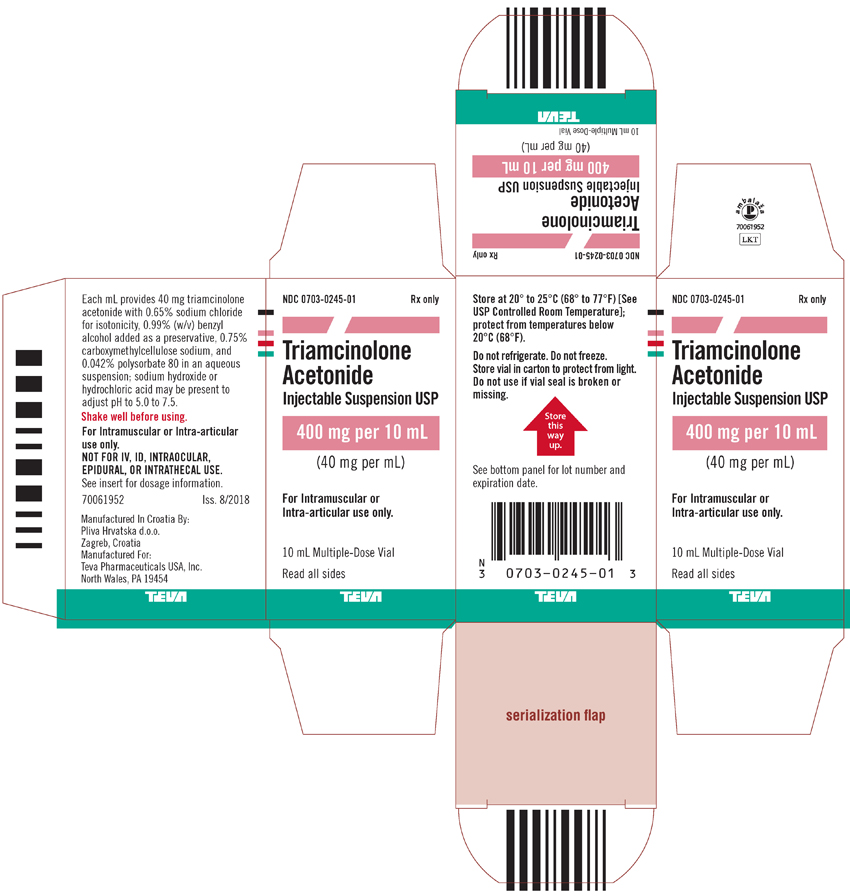
Package/Label Display PanelNDC 0703-0245-01 - Rx only - Triamcinolone Acetonide Injectable Suspension USP - 400 mg per 10 mL - (40 mg per 1 mL) For intramuscular or intra-articular use only. 10 mL Multiple-Dose Vial - Read all ...
-
INGREDIENTS AND APPEARANCEProduct Information