Label: MINOXIDIL tablet
- NDC Code(s): 0591-5642-01, 0591-5642-05, 0591-5643-01, 0591-5643-05
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNINGS
Minoxidil tablets contain the powerful antihypertensive agent, minoxidil, which may produce serious adverse effects. It can cause pericardial effusion, occasionally progressing to tamponade, and angina pectoris may be exacerbated. Minoxidil should be reserved for hypertensive patients who do not respond adequately to maximum therapeutic doses of a diuretic and two other antihypertensive agents.
In experimental animals, minoxidil caused several kinds of myocardial lesions as well as other adverse cardiac effects (see Cardiac Lesions in Animals).
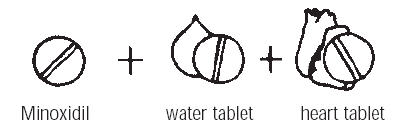
Minoxidil must be administered under close supervision, usually concomitantly with therapeutic doses of a beta-adrenergic blocking agent to prevent tachycardia and increased myocardial workload. It must also usually be given with a diuretic, frequently one acting in the ascending limb of the loop of Henle, to prevent serious fluid accumulation. Patients with malignant hypertension and those already receiving guanethidine (see WARNINGS) should be hospitalized when minoxidil is first administered so that they can be monitored to avoid too rapid, or large orthostatic, decreases in blood pressure.
Close -
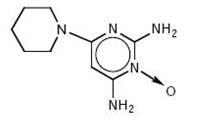
DESCRIPTIONMinoxidil tablets, USP contain minoxidil, USP an antihypertensive peripheral vasodilator. Minoxidil occurs as a white to off-white, crystalline powder, soluble in alcohol and propylene glycol ...
-
CLINICAL PHARMACOLOGY1. General Pharmacologic Properties - Minoxidil is an orally effective direct acting peripheral vasodilator that reduces elevated systolic and diastolic blood pressure by decreasing peripheral ...
-
INDICATIONS AND USAGEBecause of the potential for serious adverse effects, minoxidil tablets are indicated only in the treatment of hypertension that is symptomatic or associated with target organ damage and is not ...
-
CONTRAINDICATIONSMinoxidil tablets are contraindicated in pheochromocytoma, because it may stimulate secretion of catecholamines from the tumor through its antihypertensive action. Minoxidil is contraindicated in ...
-
WARNINGS1. Salt and Water Retention: Congestive Heart Failure—concomitant use of an adequate diuretic is required—Minoxidil tablets must usually be administered concomitantly with a diuretic adequate ...
-
PRECAUTIONS1. General Precautions - (a) Monitor fluid and electrolyte balance and body weight (see WARNINGS: Salt and Water Retention). (b) Observe patients for signs and symptoms of pericardial effusion ...
-
ADVERSE REACTIONS1. Salt and Water Retention (see WARNINGS: Concomitant Use of an Adequate Diuretic is Required)—Temporary edema developed in 7% of patients who were not edematous at the start of therapy. 2 ...
-
OVERDOSAGEThere have been only a few instances of deliberate or accidental overdosage with minoxidil tablets. One patient recovered after taking 50 mg of minoxidil together with 500 mg of a barbiturate ...
-
DOSAGE AND ADMINISTRATIONPatients over 12 years of age: The recommended initial dosage of minoxidil tablets is 5 mg of minoxidil given as a single daily dose. Daily dosage can be increased to 10, 20 and then to 40 mg in ...
-
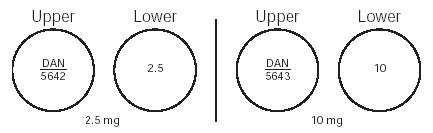
HOW SUPPLIEDMinoxidil Tablets, USP 2.5 mg are 9/32", scored, round, white tablets imprinted “DAN 5642” and “2.5” supplied in bottles of 100 NDC 0591-5642-01 and 500 NDC 0591-5642-05. Minoxidil Tablets, USP 10 ...
-
PATIENT INFORMATIONDispense with Patient Package Insert available at: www.tevausa.com/PatientPI ...
-
OTHER UNDESIRED EFFECTSMinoxidil tablets can cause other undesired effects such as nausea and/or vomiting that are annoying but not dangerous. Do not stop taking the drug because of these other undesired effects without ...
-
PRINCIPAL DISPLAY PANELNDC 0591-5642-01 - Minoxidil Tablets, USP - 2.5 mg - Rx only - 100 Tablets
-
PRINCIPAL DISPLAY PANELNDC 0591-5643-01 - Minoxidil Tablets, USP - 10 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information