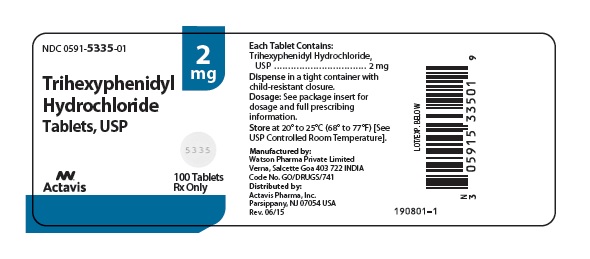
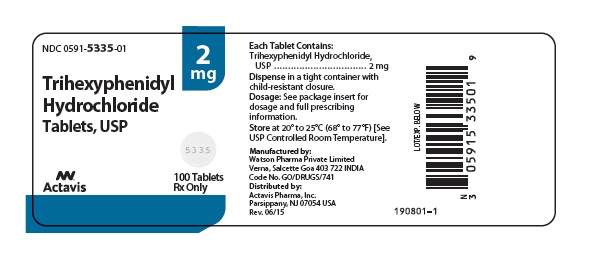
Label: TRIHEXYPHENIDYL HYDROCHLORIDE tablet
- NDC Code(s): 0591-5335-01, 0591-5335-10, 0591-5337-01, 0591-5337-10
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
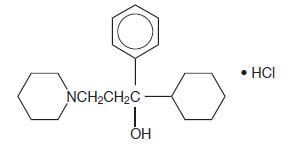
DESCRIPTIONTrihexyphenidyl hydrochloride is a synthetic antispasmodic drug available in the following forms: Tablets, 2 mg and 5 mg. It is designated chemically 1-Piperidinepropanol,α-cyclohexyl-α-phenyl- ...
-
CLINICAL PHARMACOLOGYTrihexyphenidyl HCl is the substituted piperidine salt, 3-(1-piperidyl)-1-phenylcyclohexyl-1-propanol hydrochloride, which exerts a direct inhibitory effect upon the parasympathetic nervous ...
-
INDICATIONSThis drug is indicated as an adjunct in the treatment of all forms of parkinsonism (postencephalitic, arteriosclerotic, and idiopathic). It is often useful as adjuvant therapy when treating these ...
-
WARNINGSPatients to be treated with trihexyphenidyl HCl should have a gonioscope evaluation and close monitoring of intraocular pressures at regular periodic intervals.
-
PRECAUTIONSAlthough trihexyphenidyl HCl is not contraindicated for patients with cardiac, liver, or kidney disorders, or with hypertension, such patients should be maintained under close observation. Since ...
-
ADVERSE REACTIONSMinor side effects, such as dryness of the mouth, blurring of vision, dizziness, mild nausea, or nervousness, will be experienced by 30 to 50 percent of all patients. These sensations, however ...
-
DOSAGE AND ADMINISTRATIONDosage should be individualized. The initial dose should be low and then increased gradually, especially in patients over 60 years of age. Whether trihexyphenidyl HCl may best be given before or ...
-
HOW SUPPLIEDTrihexyphenidyl hydrochloride tablets USP, 2 mg are scored, round, white, flat faced, tablets imprinted DAN DAN and 5335 supplied in bottles of 100 (NDC 0591-5335-01) and 1000 (NDC ...
-
PRINCIPAL DISPLAY PANELNDC 0591-5335-01 - Trihexyphenidyl Hydrochloride Tablets, USP 2 mg - Rx only 100 Tablets
-
PRINCIPAL DISPLAY PANELNDC 0591-5337-01 - Trihexyphenidyl Hydrochloride Tablets, USP 5 mg - Rx only 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information