Label: ALBENDAZOLE tablet, film coated
- NDC Code(s): 0591-2712-02
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALBENDAZOLE TABLETS safely and effectively. See full prescribing information for ALBENDAZOLE TABLETS. ALBENDAZOLE tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Neurocysticercosis - Albendazole tablets are indicated for the treatment of parenchymal neurocysticercosis due to active lesions caused by larval forms of the pork tapeworm, Taenia ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage - Dosing of albendazole will vary depending upon the indication. Albendazole tablets may be crushed or chewed and swallowed with a drink of water. Albendazole tablets should be taken ...
-
3 DOSAGE FORMS AND STRENGTHSTablet: 200 mg
-
4 CONTRAINDICATIONSAlbendazole is contraindicated in patients with known hypersensitivity to the benzimidazole class of compounds or any components of albendazole.
-
5 WARNINGS AND PRECAUTIONS5.1 Bone Marrow Suppression - Fatalities associated with the use of albendazole have been reported due to granulocytopenia or pancytopenia. Albendazole may cause bone marrow suppression, aplastic ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Dexamethasone - Steady-state trough concentrations of albendazole sulfoxide were about 56% higher when 8 mg dexamethasone was co-administered with each dose of albendazole (15 mg/kg/day) in 8 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal reproduction studies, albendazole may cause fetal harm when administered to a pregnant woman. However, available human data from a ...
-
10 OVERDOSAGEIn case of overdosage, symptomatic therapy and general supportive measures are recommended.
-
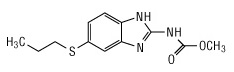
11 DESCRIPTIONAlbendazole, USP is an orally administered anthelmintic drug. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Albendazole is a synthetic, anthelmintic drug of the class benzimidazole [see Clinical Pharmacology (12.4)]. 12.3 Pharmacokinetics - Absorption - Albendazole is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies were conducted in mice and rats. No evidence of increased incidence of tumors was found in the mice ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Albendazole Tablets, USP are available as follows: 200 mg — Each white to off white, round, film-coated, unscored tablet debossed with ‘A210’ on one side and plain on the ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be advised that: Some people, particularly children, may experience difficulties swallowing the albendazole tablets whole. Take albendazole tablets with food. Advise pregnant ...
-
PRINCIPAL DISPLAY PANELNDC 0591-2712-02 - Albendazole Tablets USP - 200 mg - Rx only - 2 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information