Label: DOXYLAMINE SUCCINATE AND PYRIDOXINE HYDROCHLORIDE tablet, delayed release
- NDC Code(s): 0591-2132-01
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOXYLAMINE SUCCINATE AND PYRIDOXINE HYDROCHLORIDE DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information - Initially, take two doxylamine succinate and pyridoxine hydrochloride delayed-release tablets orally at bedtime (Day 1). If this dose adequately controls symptoms the ...
-
3 DOSAGE FORMS AND STRENGTHS
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are white to off white, unscored, round, biconvex film coated tablets, plain on both sides containing 10 mg doxylamine ...
-
4 CONTRAINDICATIONS
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are contraindicated in women with any of the following conditions: Known hypersensitivity to doxylamine succinate, other ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Activities Requiring Mental Alertness - Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets may cause somnolence due to the anticholinergic properties of doxylamine ...
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling: Somnolence [see Warnings and Precautions (5.1)] Falls or other accidents resulting from the effect of the combined use of ...
-
7 DRUG INTERACTIONS
7.1 Drug Interactions - Use of doxylamine succinate and pyridoxine hydrochloride delayed-release tablets is contraindicated in women who are taking monoamine oxidase inhibitors (MAOIs), which ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are intended for the treatment of nausea and vomiting of pregnancy in women who do not ...
-
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose - Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets is a delayed-release formulation, therefore, signs and symptoms of intoxication may ...
-
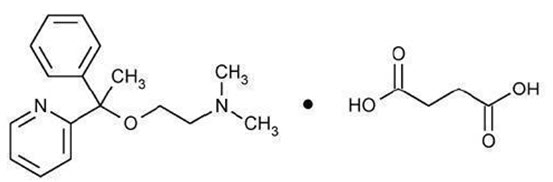
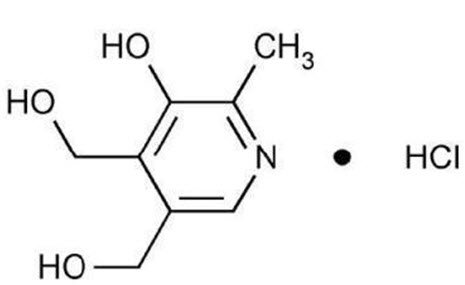
11 DESCRIPTION
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are round, unscored, white to off white, biconvex film-coated, delayed-release tablets containing 10 mg of doxylamine ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism of action of doxylamine succinate and pyridoxine hydrochloride delayed-release tablets is unknown. 12.3 Pharmacokinetics - The pharmacokinetics of ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility - Carcinogenicity - Two-year carcinogenicity studies in rats and mice have been conducted with doxylamine succinate. Doxylamine ...
-
14 CLINICAL STUDIES
A double-blind, randomized, multi-center, placebo-controlled study was conducted to support the safety and efficacy of doxylamine succinate and pyridoxine hydrochloride delayed-release tablets in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How supplied - Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets 100 bottle count is supplied in a high-density polyethylene bottle with a polypropylene ...
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information) Somnolence and Severe Drowsiness - Inform women to avoid engaging in activities requiring complete mental alertness, such as driving or ...
-
SPL UNCLASSIFIED SECTIONDispense with Patient Package Insert available at: www.tevausa.com/PatientPI
-
Patient InformationDoxylamine Succinate (dox il′ a meen sux′ i nate) and Pyridoxine Hydrochloride (pir′′ i dox′ een hye′′ droe klor′ ide) Delayed-release Tablets - What are doxylamine succinate and pyridoxine ...
-
Principal Display PanelNDC 0591-2132-01 - Doxylamine Succinate and Pyridoxine Hydrochloride Delayed-Release Tablets - 10 mg/10 mg - PHARMACIST: Dispense with the accompany Patient Information to each patient. WARNING: Swallow ...
-
INGREDIENTS AND APPEARANCEProduct Information