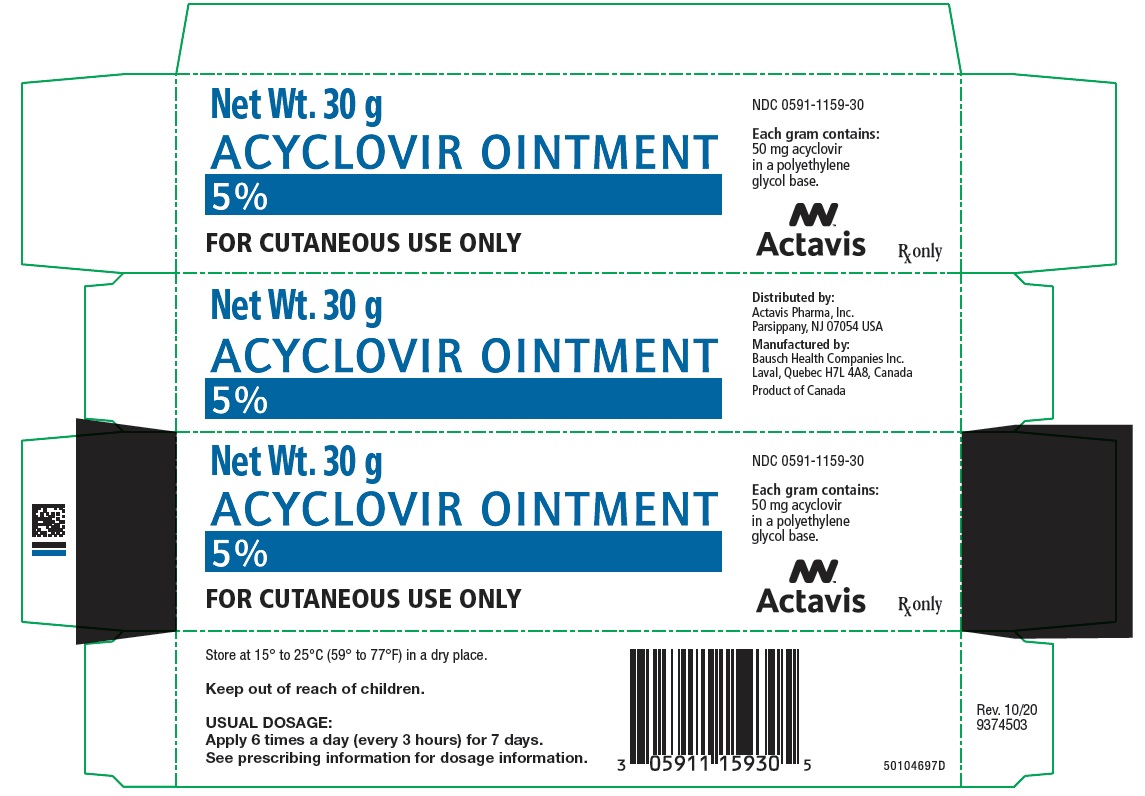
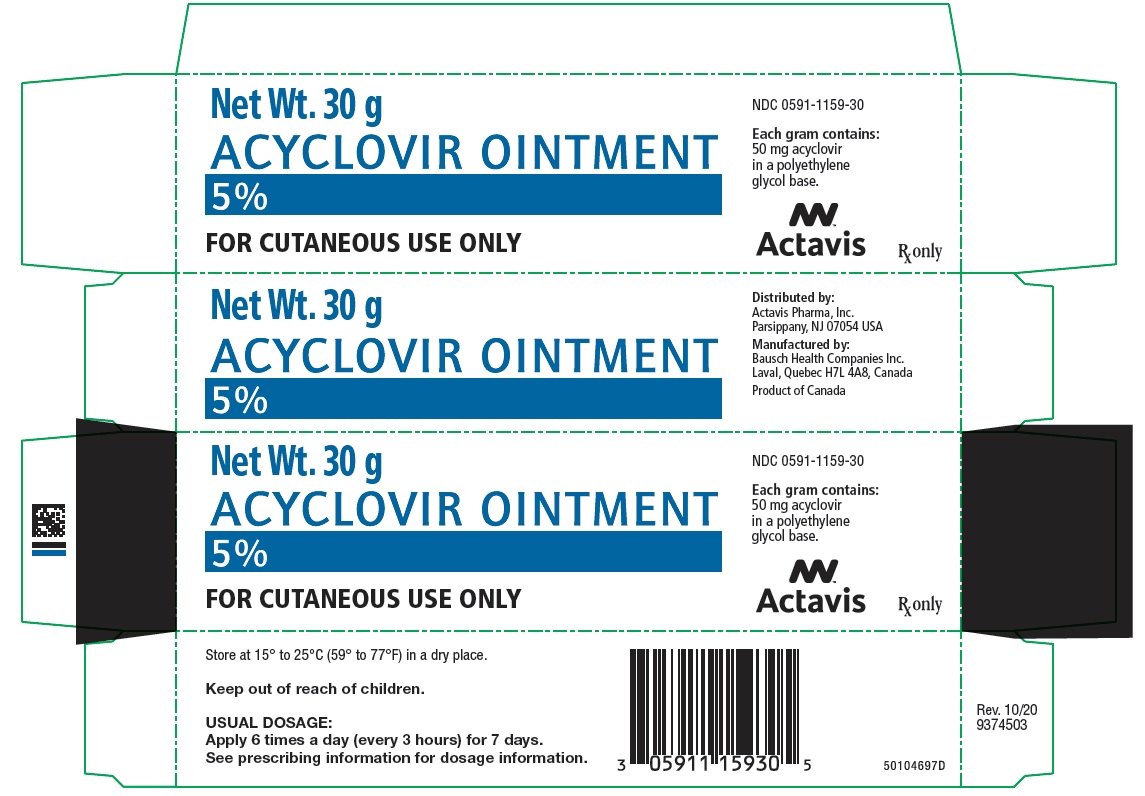
Label: ACYCLOVIR ointment
- NDC Code(s): 0591-1159-30
- Packager: ACTAVIS PHARMA, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated October 31, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONAcyclovir - Ointment 5%
-
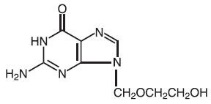
DESCRIPTIONAcyclovir is a synthetic nucleoside analogue active against herpes viruses. Acyclovir Ointment 5% is a formulation for topical administration. Each gram of Acyclovir Ointment 5% contains 50 mg of ...
-
VIROLOGYMechanism of Antiviral Action: Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and ...
-
CLINICAL PHARMACOLOGYTwo clinical pharmacology studies were performed with acyclovir ointment 5% in immunocompromised adults at risk of developing mucocutaneous HSV infections or with localized varicella zoster ...
-
CLINICAL TRIALSIn clinical trials of initial genital herpes infections, Acyclovir Ointment 5% has shown a decrease in healing time and, in some cases, a decrease in duration of viral shedding and duration of ...
-
INDICATIONS AND USAGEAcyclovir Ointment 5% is indicated in the management of initial genital herpes and in limited non-lifethreatening mucocutaneous HSV infections in immunocompromised patients.
-
CONTRAINDICATIONSAcyclovir Ointment 5% is contraindicated in patients who develop hypersensitivity to the components of the formulation.
-
WARNINGSAcyclovir Ointment 5% is intended for cutaneous use only and should not be used in the eye.
-
PRECAUTIONSGeneral: The recommended dosage, frequency of applications, and length of treatment should not be exceeded (see DOSAGE AND ADMINISTRATION). There are no data to support the use of Acyclovir ...
-
ADVERSE REACTIONSIn the controlled clinical trials, mild pain (including transient burning and stinging) was reported by about 30% of patients in both the active and placebo arms; treatment was discontinued in two ...
-
OVERDOSAGEOverdosage by topical application of Acyclovir Ointment 5% is unlikely because of limited transcutaneous absorption (see CLINICAL PHARMACOLOGY).
-
DOSAGE AND ADMINISTRATIONApply sufficient quantity to adequately cover all lesions every 3 hours, 6 times per day for 7 days. The dose size per application will vary depending upon the total lesion area but should ...
-
HOW SUPPLIEDEach gram of Acyclovir Ointment 5% contains 50 mg acyclovir in a polyethylene glycol base. It is supplied as follows: 30 g tubes NDC 0591-1159-30 - Store at 15° to 25°C (59° to ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Actavis Pharma, Inc. Parsippany, NJ 07054 USA - Manufactured by: Bausch Health Companies Inc. Laval, Quebec H7L 4A8, Canada - ZOVIRAX is a trademark of GlaxoSmithKline LLC used ...
-
PRINCIPAL DISPLAY PANEL - 30 g Tube CartonNet Wt. 30 g - ACYCLOVIR OINTMENT - 5% FOR CUTANEOUS USE ONLY - NDC 0591-1159-30 - Each gram contains: 50 mg acyclovir - in a polyethylene - glycol base. Actavis - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information