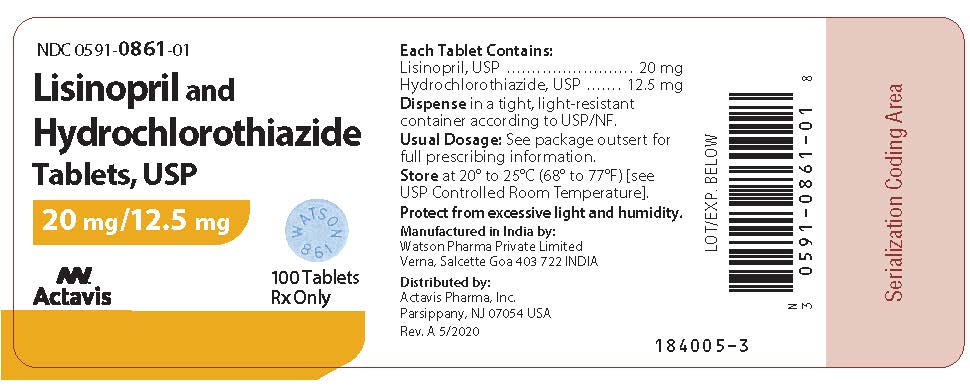
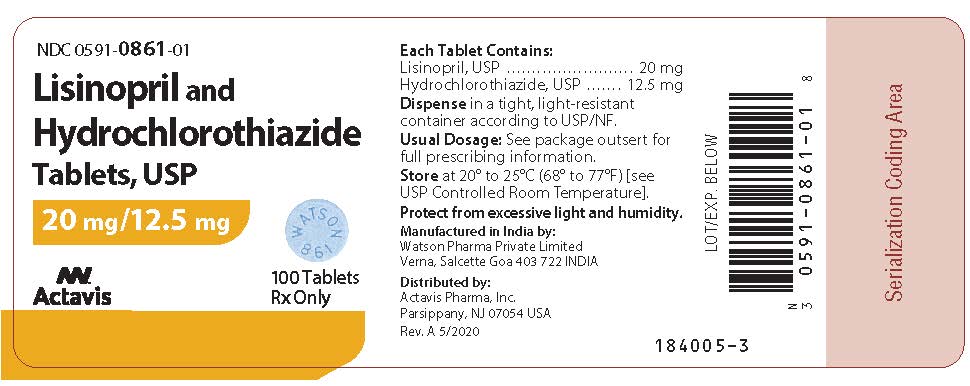
Label: LISINOPRIL AND HYDROCHLOROTHIAZIDE tablet
-
NDC Code(s):
0591-0860-01,
0591-0860-05,
0591-0861-01,
0591-0861-05, view more0591-0862-01, 0591-0862-05
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BOXED WARNING (What is this?)
-
DESCRIPTION
Lisinopril and Hydrochlorothiazide Tablets, USP combine an angiotensin converting enzyme inhibitor, lisinopril, USP and a diuretic, hydrochlorothiazide, USP. Lisinopril, USP a synthetic peptide ...
Lisinopril and Hydrochlorothiazide Tablets, USP combine an angiotensin converting enzyme inhibitor, lisinopril, USP and a diuretic, hydrochlorothiazide, USP.
Lisinopril, USP a synthetic peptide derivative, is an oral long-acting angiotensin converting enzyme inhibitor. It is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5•2H2O and its structural formula is:
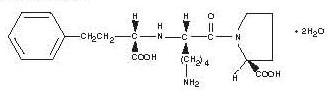
Lisinopril, USP is a white to off-white, crystalline powder, with a molecular weight of 441.52. It is soluble in water, sparingly soluble in methanol, and practically insoluble in ethanol.
Hydrochlorothiazide, USP is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
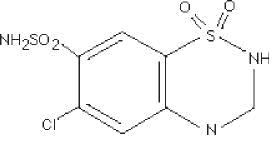
Hydrochlorothiazide, USP is a white, or practically white, crystalline powder with a molecular weight of 297.74, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
Lisinopril and Hydrochlorothiazide Tablets, USP are available for oral use in three tablet combinations of lisinopril with hydrochlorothiazide: Lisinopril and Hydrochlorothiazide Tablets USP, 10/12.5, which contains 10 mg lisinopril and 12.5 mg hydrochlorothiazide, USP; Lisinopril and Hydrochlorothiazide Tablets USP, 20/12.5, which contains 20 mg lisinopril, USP and 12.5 mg hydrochlorothiazide, USP; and Lisinopril and Hydrochlorothiazide Tablets USP, 20/25, which contains 20 mg lisinopril, USP and 25 mg hydrochlorothiazide, USP. Inactive ingredients are colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, and pregelatinized corn starch. The 10 mg/12.5 mg and 20 mg/25 mg tablets also contain FD&C Red #40 Aluminum Lake. The 20 mg/12.5 mg tablet also contains FD&C Blue #2 Aluminum Lake.
Close -
CLINICAL PHARMACOLOGY
Lisinopril-Hydrochlorothiazide - As a result of its diuretic effects, hydrochlorothiazide increases plasma renin activity, increases aldosterone secretion, and decreases serum potassium ...
Lisinopril-Hydrochlorothiazide
As a result of its diuretic effects, hydrochlorothiazide increases plasma renin activity, increases aldosterone secretion, and decreases serum potassium. Administration of lisinopril blocks the renin- angiotensin-aldosterone axis and tends to reverse the potassium loss associated with the diuretic.
In clinical studies, the extent of blood pressure reduction seen with the combination of lisinopril and hydrochlorothiazide was approximately additive. The Lisinopril and Hydrochlorothiazide Tablets 10/12.5 combination worked equally well in Black and Caucasian patients. The Lisinopril and Hydrochlorothiazide Tablets 20/12.5 and Lisinopril and Hydrochlorothiazide Tablets 20/25 (a previously - marketed strength) combinations appeared somewhat less effective in Black patients, but relatively few Black patients were studied. In most patients, the antihypertensive effect of Lisinopril and Hydrochlorothiazide Tablets was sustained for at least 24 hours.
In a randomized, controlled comparison, the mean antihypertensive effects of Lisinopril and Hydrochlorothiazide Tablets 20/12.5 and Lisinopril and Hydrochlorothiazide Tablets 20/25 were similar, suggesting that many patients who respond adequately to the latter combination may be controlled with Lisinopril and Hydrochlorothiazide Tablets 20/12.5 (see DOSAGE AND ADMINISTRATION).
Concomitant administration of lisinopril and hydrochlorothiazide has little or no effect on the bioavailability of either drug. The combination tablet is bioequivalent to concomitant administration of the separate entities.
Lisinopril
Mechanism of Action
Lisinopril inhibits angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. Inhibition of ACE results in decreased plasma angiotensin II which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity. In hypertensive patients with normal renal function treated with lisinopril alone for up to 24 weeks, the mean increase in serum potassium was less than 0.1 mEq/L; however, approximately 15 percent of patients had increases greater than 0.5 mEq/L and approximately six percent had a decrease greater than0.5 mEq/L. In the same study, patients treated with lisinopril plus a thiazide diuretic showed essentially no change in serum potassium (see PRECAUTIONS).
ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of lisinopril remains to be elucidated.
While the mechanism through which lisinopril lowers blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, lisinopril is antihypertensive even in patients with low-renin hypertension. Although lisinopril was antihypertensive in all races studied, Black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to lisinopril monotherapy than non-Black patients.
Pharmacokinetics and Metabolism
Following oral administration of lisinopril, peak serum concentrations occur within about 7 hours. Declining serum concentrations exhibit a prolonged terminal phase which does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose. Lisinopril does not appear to be bound to other serum proteins.
Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of absorption of lisinopril is approximately 25 percent, with large intersubject variability (6 to 60 percent) at all doses tested (5 to 80 mg). Lisinopril absorption is not influenced by the presence of food in the gastrointestinal tract.
Upon multiple dosing, lisinopril exhibits an effective half-life of accumulation of 12 hours.
Impaired renal function decreases elimination of lisinopril, which is excreted principally through the kidneys, but this decrease becomes clinically important only when the glomerular filtration rate is below
30 mL/min. Above this glomerular filtration rate, the elimination half-life is little changed. With greater impairment, however, peak and trough lisinopril levels increase, time to peak concentration increases and time to attain steady state is prolonged. Older patients, on average, have (approximately doubled) higher blood levels and area under the plasma concentration time curve (AUC) than younger patients (see DOSAGE AND ADMINISTRATION). Lisinopril can be removed by hemodialysis.
Studies in rats indicate that lisinopril crosses the blood-brain barrier poorly. Multiple doses of lisinopril in rats do not result in accumulation in any tissues. However, milk of lactating rats contains radioactivity following administration of 14C lisinopril. By whole body autoradiography, radioactivity was found in the placenta following administration of labeled drug to pregnant rats, but none was found in the fetuses.
Pharmacodynamics
Administration of lisinopril to patients with hypertension results in a reduction of supine and standing blood pressure to about the same extent with no compensatory tachycardia. Symptomatic postural hypotension is usually not observed although it can occur and should be anticipated in volume and/or salt-depleted patients (see WARNINGS).
In most patients studied, onset of antihypertensive activity was seen at one hour after oral administration of an individual dose of lisinopril, with peak reduction of blood pressure achieved by six hours.
In some patients achievement of optimal blood pressure reduction may require two to four weeks of therapy.
At recommended single daily doses, antihypertensive effects have been maintained for at least 24 hours after dosing, although the effect at 24 hours was substantially smaller than the effect six hours after dosing.
The antihypertensive effects of lisinopril have continued during long-term therapy. Abrupt withdrawal of lisinopril has not been associated with a rapid increase in blood pressure; nor with a significant overshoot of pretreatment blood pressure.
In hemodynamic studies in patients with essential hypertension, blood pressure reduction was accompanied by a reduction in peripheral arterial resistance with little or no change in cardiac output and in heart rate. In a study in nine hypertensive patients, following administration of lisinopril, there was an increase in mean renal blood flow that was not significant. Data from several small studies are inconsistent with respect to the effect of lisinopril on glomerular filtration rate in hypertensive patients with normal renal function, but suggest that changes, if any, are not large.
In patients with renovascular hypertension lisinopril has been shown to be well tolerated and effective in controlling blood pressure (see PRECAUTIONS).
Hydrochlorothiazide
The mechanism of the antihypertensive effect of thiazides is unknown. Thiazides do not usually affect normal blood pressure.
Hydrochlorothiazide is a diuretic and antihypertensive. It affects the distal renal tubular mechanism of electrolyte reabsorption. Hydrochlorothiazide increases excretion of sodium and chloride in approximately equivalent amounts. Natriuresis may be accompanied by some loss of potassium and bicarbonate.
After oral use diuresis begins within two hours, peaks in about four hours and lasts about 6 to 12 hours. Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. When plasma levels have
been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours. At least 61 percent of the oral dose is eliminated unchanged within 24 hours. Hydrochlorothiazide crosses the placental but not the blood-brain barrier.
Close -
INDICATIONS AND USAGE
Lisinopril and Hydrochlorothiazide Tablets is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular ...
Lisinopril and Hydrochlorothiazide Tablets is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including lisinopril and hydrochlorothiazide.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
These fixed-dose combinations are not indicated for initial therapy (see DOSAGE AND ADMINISTRATION).
In using Lisinopril and Hydrochlorothiazide Tablets, consideration should be given to the fact that an angiotensin converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease, and that available data are insufficient to show that lisinopril does not have a similar risk (see WARNINGS).
In considering use of Lisinopril and Hydrochlorothiazide Tablets, it should be noted that Black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to non- Blacks (see WARNINGS, Head and Neck Angioedema).
Close -
CONTRAINDICATIONS
Lisinopril and Hydrochlorothiazide Tablets is contraindicated in patients who are hypersensitive to any component of this product and in patients with a history of angioedema related to previous ...
Lisinopril and Hydrochlorothiazide Tablets is contraindicated in patients who are hypersensitive to any component of this product and in patients with a history of angioedema related to previous treatment with an angiotensin converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema. Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
Lisinopril and Hydrochlorothiazide Tablets is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer Lisinopril and Hydrochlorothiazide Tablets within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor (see WARNINGS).
Do not coadminister aliskiren with Lisinopril and Hydrochlorothiazide Tablets in patients with diabetes.
Close -
WARNINGS
General - Lisinopril - Anaphylactoid and Possibly Related Reactions Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including ...
General
Lisinopril
Anaphylactoid and Possibly Related Reactions Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including Lisinopril and Hydrochlorothiazide Tablets) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis and/or larynx has been reported rarely in patients treated with angiotensin converting enzyme inhibitors, including lisinopril. This may occur at any time during treatment. ACE inhibitors have been associated with a higher rate of angioedema in Black than in non-Black patients. In such cases Lisinopril and Hydrochlorothiazide Tablets should be promptly discontinued and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms has occurred. Even in those instances where swelling of only the tongue is involved, without respiratory distress, patients may require prolonged observation since treatment with antihistamines and corticosteroids may not be sufficient. Very rarely, fatalities have been reported due to angioedema associated with laryngeal edema or tongue edema. Patients with involvement of the tongue, glottis or larynx are likely to experience airway obstruction, especially those with a history of airway surgery. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided (see ADVERSE REACTIONS).
Patients with a history of angioedema unrelated to ACE-inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor (see also INDICATIONS AND USAGE and CONTRAINDICATIONS).
Patients receiving coadministration of ACE inhibitor and mTOR (mammalian target of rapamycin) inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema (see PRECAUTIONS).
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid reactions during desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid reactions during membrane exposure: Sudden and potentially life-threatening anaphylactoid reactions have been reported in some patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. In such patients, dialysis must be stopped immediately, and aggressive therapy for anaphylactoid reactions must be initiated. Symptoms have not been relieved by antihistamines in these situations. In these patients, consideration should be given to using a different type of dialysis membrane or a different class of antihypertensive agent. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hypotension and Related Effects Excessive hypotension was rarely seen in uncomplicated hypertensive patients but is a possible consequence of lisinopril use in salt/volume-depleted persons, such as those treated vigorously with diuretics or patients on dialysis (see PRECAUTIONS, Drug Interactions and ADVERSE REACTIONS).
Syncope has been reported in 0.8 percent of patients receiving Lisinopril and Hydrochlorothiazide Tablets. In patients with hypertension receiving lisinopril alone, the incidence of syncope was 0.1 percent. The overall incidence of syncope may be reduced by proper titration of the individual components(see PRECAUTIONS, Drug Interactions, ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION).
In patients with severe congestive heart failure, with or without associated renal insufficiency, excessive hypotension has been observed and may be associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death. Because of the potential fall in blood pressure in these patients, therapy should be started under very close medical supervision. Such patients should be followed closely for the first two weeks of treatment and whenever the dose of lisinopril and/or diuretic is increased. Similar considerations apply to patients with ischemic heart or cerebrovascular disease in whom an excessive fall in blood pressure could result in a myocardial infarction or cerebrovascular accident.
If hypotension occurs, the patient should be placed in supine position and, if necessary, receive an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further doses which usually can be given without difficulty once the blood pressure has increased after volume expansion.
Neutropenia/Agranulocytosis Another angiotensin converting enzyme inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression, rarely in uncomplicated patients but more frequently in patients with renal impairment, especially if they also have a collagen vascular disease. Available data from clinical trials of lisinopril are insufficient to show that lisinopril does not cause agranulocytosis at similar rates. Marketing experience has revealed rare cases of neutropenia and bone marrow depression in which a causal relationship to lisinopril cannot be excluded. Periodic monitoring of white blood cell counts in patients with collagen vascular disease and renal disease should be considered.
Hepatic Failure Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice or hepatitis and progresses to fulminant hepatic necrosis, and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Hydrochlorothiazide
Thiazides should be used with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Sensitivity reactions may occur in patients with or without a history of allergy or bronchial asthma. The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
Lithium generally should not be given with thiazides (see PRECAUTIONS, Drug Interactions, Lisinopril and Hydrochlorothiazide).
Acute Myopia and Secondary Angle-Closure Glaucoma: Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Lisinopril and Hydrochlorothiazide Tablets as soon as possible. These adverse outcomes are usually associated with the use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative therapy to drugs affecting the renin- angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Lisinopril and Hydrochlorothiazide Tablets, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Lisinopril and Hydrochlorothiazide Tablets for hypotension, oliguria, and hyperkalemia (see PRECAUTIONS, Pediatric Use).
Lisinopril-Hydrochlorothiazide
Teratogenicity studies were conducted in mice and rats with up to 90 mg/kg/day of lisinopril in combination with 10 mg/kg/day of hydrochlorothiazide. This dose of lisinopril is 5 times (in mice) and 10 times (in rats) the maximum recommended human daily dose (MRHDD) when compared on a body surface area basis (mg/m2); the dose of hydrochlorothiazide is 0.9 times (in mice) and 1.8 times (in rats) the MRHDD. Maternal or fetotoxic effects were not seen in mice with the combination. In rats decreased maternal weight gain and decreased fetal weight occurred down to 3/10 mg/kg/day (the lowest dose tested). Associated with the decreased fetal weight was a delay in fetal ossification. The decreased fetal weight and delay in fetal ossification were not seen in saline-supplemented animals given 90/10 mg/kg/day.
No teratogenic effects of lisinopril were seen in studies of pregnant mice, rats, and rabbits. On a body surface area basis, the doses used were up 55 times, 33 times, and 0.15 times, respectively, the MRHDD.
Hydrochlorothiazide
Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3000 and 1000 mg/kg/day, respectively, provided no evidence of harm to the fetus. These doses are more than 150 times the MRHDD on a body surface area basis. Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia and possibly other adverse reactions that have occurred in adults.
Close -
PRECAUTIONS
General - Lisinopril - Aortic Stenosis/Hypertrophic Cardiomyopathy: As with all vasodilators, lisinopril should be given with caution to patients with obstruction in the outflow tract of ...
General
Lisinopril
Aortic Stenosis/Hypertrophic Cardiomyopathy:
As with all vasodilators, lisinopril should be given with caution to patients with obstruction in the outflow tract of the left ventricle.
Impaired Renal Function:
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe congestive heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with angiotensin converting enzyme inhibitors, including lisinopril, may be associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death.
In hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine may occur. Experience with another angiotensin converting enzyme inhibitor suggests that these increases are usually reversible upon discontinuation of lisinopril and/or diuretic therapy. In such patients renal function should be monitored during the first few weeks of therapy.
Some hypertensive patients with no apparent pre-existing renal vascular disease have developed increases in blood urea and serum creatinine, usually minor and transient, especially when lisinopril has been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction of lisinopril and/or discontinuation of the diuretic may be required.
Evaluation of the hypertensive patient should always include assessment of renal function (see DOSAGE AND ADMINISTRATION).
Hyperkalemia:
In clinical trials hyperkalemia (serum potassium greater than 5.7 mEq/L) occurred in approximately 1.4 percent of hypertensive patients treated with lisinopril plus hydrochlorothiazide. In most cases these were isolated values which resolved despite continued therapy. Hyperkalemia was not a cause of discontinuation of therapy. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes. Hyperkalemia can cause serious, sometimes fatal, arrhythmias. Lisinopril and Hydrochlorothiazide Tablets should be used cautiously, if at all, with these agents and with frequent monitoring of serum potassium (see PRECAUTIONS, Drug Interactions).
Cough:
Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough.
Surgery/Anesthesia:
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, lisinopril may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Hydrochlorothiazide
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance, irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present, or after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability). Because lisinopril reduces the production of aldosterone, concomitant therapy with lisinopril attenuates the diuretic-induced potassium loss (see PRECAUTIONS, Drug Interactions, Agents Increasing Serum Potassium).
Although any chloride deficit is generally mild and usually does not require specific treatment, except under extraordinary circumstances (as in liver disease or renal disease), chloride replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt except in rare instances when the hyponatremia is life- threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required. Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post sympathectomy patient.
If progressive renal impairment becomes evident consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Information for Patients
Angioedema: Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin converting enzyme inhibitors, including lisinopril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Symptomatic Hypotension: Patients should be cautioned to report lightheadedness especially during the first few days of therapy. If actual syncope occurs, the patients should be told to discontinue the drug until they have consulted with the prescribing physician.
All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult with their physician.
Hyperkalemia: Patients should be told not to use salt substitutes containing potassium without consulting their physician.
Neutropenia: Patients should be told to report promptly any indication of infection (e.g., sore throat, fever) which may be a sign of neutropenia.
Pregnancy: Female patients of childbearing age should be told about the consequences of exposure to Lisinopril and Hydrochlorothiazide Tablets during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
Non-melanoma Skin Cancer: Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Drug Interactions
Lisinopril
Hypotension — Patients on Diuretic Therapy: Patients on diuretics, and especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with lisinopril. The possibility of hypotensive effects with lisinopril can be minimized by either discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with lisinopril. If it is necessary to continue the diuretic, initiate therapy with lisinopril at a dose of 5 mg daily, and provide close medical supervision after the initial dose for at least two hours and until blood pressure has stabilized for at least an additional hour (see WARNINGS and DOSAGE AND ADMINISTRATION). When a diuretic is added to the therapy of a patient receiving lisinopril, an additional antihypertensive effect is usually observed (see DOSAGE AND ADMINISTRATION).
Non-steroidal Anti-inflammatory Agents Including Selective Cyclooxygenase-2 (COX-2) Inhibitors: In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including lisinopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving lisinopril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including lisinopril, may be attenuated by NSAIDs.
Dual Blockade of the Renin-Angiotensin System (RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or direct renin inhibitors (such as aliskiren) is associated with increased risk of hypotension, syncope, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy.
The VA NEPHRON trial enrolled 1448 patients with type 2 diabetes, elevated urinary-albumin to-creatinine ratio, and decreased estimated glomerular filtration rate (GFR 30 to 89.9 ml/min), randomized them to lisinopril or placebo on a background of losartan therapy and followed them for a median of 2.2 years. Patients receiving the combination of losartan and lisinopril did not obtain any additional benefit compared to monotherapy for the combined endpoint of decline in GFR, end state renal disease, or death, but experienced an increased incidence of hyperkalemia and acute kidney injury compared with the monotherapy group.
In general, avoid combined use of RAS inhibitors. Monitor blood pressure, renal function, and electrolytes in patients on Lisinopril and Hydrochlorothiazide Tablets and other agents that affect the RAS.
Do not coadminister aliskiren with Lisinopril and Hydrochlorothiazide Tablets in patients with diabetes. Avoid use of aliskiren with PRINZIDE in patients with renal impairment (GFR <60 ml/min).
Other Agents: Lisinopril has been used concomitantly with nitrates and/or digoxin without evidence of clinically significant adverse interactions. No meaningful clinically important pharmacokinetic interactions occurred when lisinopril was used concomitantly with propranolol, digoxin, or hydrochlorothiazide. The presence of food in the stomach does not alter the bioavailability of lisinopril.
Agents Increasing Serum Potassium: Lisinopril attenuates potassium loss caused by thiazide-type diuretics. Use of lisinopril with potassium-sparing diuretics (e.g., spironolactone, eplerenone, triamterene, or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated, because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium.
Lithium: Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. It is recommended that serum lithium levels be monitored frequently if lisinopril is administered concomitantly with lithium.
Gold: Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including Lisinopril and Hydrochlorothiazide Tablets.
mTOR (mammalian target of rapamycin) inhibitors: Patients receiving coadministration of ACE inhibitor and mTOR inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema (seeWARNINGS).
Neprilysin Inhibitors: Patients taking concomitant neprilysin inhibitors may be at increased risk for angioedema (see WARNINGS).
Hydrochlorothiazide
When administered concurrently the following drugs may interact with thiazide diuretics.
Alcohol, barbiturates, or narcotics — potentiation of orthostatic hypotension may occur.
Antidiabetic drugs (oral agents and insulin) — dosage adjustment of the antidiabetic drug may be required.
Other antihypertensive drugs — additive effect or potentiation.
Cholestyramine and colestipol resins — Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85 and 43 percent, respectively.
Corticosteroids, ACTH — intensified electrolyte depletion, particularly hypokalemia.
Pressor amines (e.g., norepinephrine) — possible decreased response to pressor amines but not sufficient to preclude their use.
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine) — possible increased responsiveness to the muscle relaxant.
Lithium — should not generally be given with diuretics. Diuretic agents reduce the renal clearance of lithium and add a high risk of lithium toxicity. Refer to the package insert for lithium preparations before use of such preparations with Lisinopril and Hydrochlorothiazide Tablets.
Non-steroidal Anti-inflammatory Drugs — In some patients, the administration of a non-steroidal anti- inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium- sparing and thiazide diuretics. Therefore, when Lisinopril and Hydrochlorothiazide Tablets and non- steroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of Lisinopril and Hydrochlorothiazide Tablets is obtained.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lisinopril-Hydrochlorothiazide
Lisinopril in combination with hydrochlorothiazide was not mutagenic in a microbial mutagen test using Salmonella typhimurium (Ames test) or Escherichia coli with or without metabolic activation or in a forward mutation assay using Chinese hamster lung cells. Lisinopril-hydrochlorothiazide did not produce DNA single strand breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, it did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
Lisinopril
There was no evidence of a tumorigenic effect when lisinopril was administered orally for 105 weeks to male and female rats at doses up to 90 mg/kg/day or for 92 weeks to male and female mice at doses up to 135 mg/kg/day. These doses are 10 times and 7 times, respectively, the maximum recommended human daily dose (MRHDD) when compared on a body surface area basis.
Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
There were no adverse effects on reproductive performance in male and female rats treated with up to 300 mg/kg/day of lisinopril (33 times the MRHDD when compared on a body surface area basis).
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice at doses of up to approximately 600 mg/kg/day (53 times the MRHDD when compared on a body surface area basis) or in male and female rats at doses of up to approximately 100 mg/kg/day (18 times the MRHDD when compared on a body surface area basis). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 mcg/mL, and in the Aspergillus nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to conception and throughout gestation. In mice and rats these doses are 9 times and 0.7 times, respectively, the MRHDD when compared on a body surface area basis.
Nursing Mothers
It is not known whether lisinopril is secreted in human milk. However, milk of lactating rats contains radioactivity following administration of 14C lisinopril. In another study, lisinopril was present in rat milk at levels similar to plasma levels in the dams. Thiazides do appear in human milk. Because of the potential for serious reactions in nursing infants from ACE inhibitors and hydrochlorothiazide, a decision should be made whether to discontinue nursing or to discontinue Lisinopril and Hydrochlorothiazide Tablets, taking into account the importance of the drug to the mother.
Pediatric Use
Neonates with a history of in utero exposure to Lisinopril and Hydrochlorothiazide Tablets:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Lisinopril, which crosses the placenta, has been removed from neonatal circulation by peritoneal dialysis with some clinical benefit, and theoretically may be removed by exchange transfusion, although there is no experience with the latter procedure.
CloseGeriatric Use
Clinical studies of Lisinopril and Hydrochlorothiazide Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. In a multiple-dose pharmacokinetic study in elderly versus young hypertensive patients using the lisinopril/hydrochlorothiazide combination, area under the plasma concentration time curve (AUC) increased approximately 120% for lisinopril and approximately 80% for hydrochlorothiazide in older patients.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. Evaluation of the hypertensive patient should always include assessment of renal function (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Lisinopril and Hydrochlorothiazide Tablets have been evaluated for safety in 930 patients, including 100 patients treated for 50 weeks or more. In clinical trials with Lisinopril and ...
Lisinopril and Hydrochlorothiazide Tablets have been evaluated for safety in 930 patients, including 100 patients treated for 50 weeks or more.
In clinical trials with Lisinopril and Hydrochlorothiazide Tablets no adverse experiences peculiar to this combination drug have been observed. Adverse experiences that have occurred have been limited to those that have been previously reported with lisinopril or hydrochlorothiazide.
The most frequent clinical adverse experiences in controlled trials (including open label extensions) with any combination of lisinopril and hydrochlorothiazide were: dizziness (7.5 percent), headache (5.2 percent), cough (3.9 percent), fatigue (3.7 percent) and orthostatic effects (3.2 percent), all of which were more common than in placebo-treated patients. Generally, adverse experiences were mild and transient in nature; but see WARNINGS regarding angioedema and excessive hypotension or syncope.
Discontinuation of therapy due to adverse effects was required in 4.4 percent of patients, principally because of dizziness, cough, fatigue and muscle cramps.
Adverse experiences occurring in greater than one percent of patients treated with lisinopril plus hydrochlorothiazide in controlled clinical trials are shown below.
Percent of Patients in Controlled Studies Lisinopril and Hydrochlorothiazide
(n=930)
Incidence
(discontinuation)Placebo
(n=207)
IncidenceDizziness 7.5 (0.8) 1.9 Headache 5.2 (0.3) 1.9 Cough 3.9 (0.6) 1.0 Fatigue 3.7 (0.4) 1.0 Orthostatic Effects 3.2 (0.1) 1.0 Diarrhea 2.5 (0.2) 2.4 Nausea 2.2 (0.1) 2.4 Upper Respiratory Infection 2.2 (0.0) 0.0 Muscle Cramps 2.0 (0.4) 0.5 Asthenia 1.8 (0.2) 1.0 Paresthesia 1.5 (0.1) 0.0 Hypotension 1.4 (0.3) 0.5 Vomiting 1.4 (0.1) 0.5 Dyspepsia 1.3 (0.0) 0.0 Rash 1.2 (0.1) 0.5 Impotence 1.2 (0.3) 0.0 Clinical adverse experiences occurring in 0.3 to 1.0 percent of patients in controlled trials included:
Body as a Whole: Chest pain, abdominal pain, syncope, chest discomfort, fever, trauma, virus infection. Cardiovascular: Palpitation, orthostatic hypotension. Digestive: Gastrointestinal cramps, dry mouth, constipation, heartburn. Musculoskeletal: Back pain, shoulder pain, knee pain, back strain, myalgia, foot pain. Nervous/Psychiatric: Decreased libido, vertigo, depression, somnolence. Respiratory: Common cold, nasal congestion, influenza, bronchitis, pharyngeal pain, dyspnea, pulmonary congestion, chronic sinusitis, allergic rhinitis, pharyngeal discomfort. Skin: Flushing, pruritus, skin inflammation, diaphoresis. Special Senses: Blurred vision, tinnitus, otalgia. Urogenital: Urinary tract infection.
Angioedema: Angioedema has been reported in patients receiving PRINZIDE, with an incidence higher in Black than in non-Black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with PRINZIDE should be discontinued and appropriate therapy instituted immediately. In rare cases, intestinal angioedema has been reported with angiotensin converting enzyme inhibitors including lisinopril (see WARNINGS).
Hypotension: In clinical trials, adverse effects relating to hypotension occurred as follows: hypotension (1.4 percent), orthostatic hypotension (0.5 percent), other orthostatic effects (3.2 percent). In addition syncope occurred in 0.8 percent of patients (see WARNINGS).
Cough: See PRECAUTIONS, Cough.
Clinical Laboratory Test Findings
Serum Electrolytes: See PRECAUTIONS.
Creatinine, Blood Urea Nitrogen: Minor reversible increases in blood urea nitrogen and serum creatinine were observed in patients with essential hypertension treated with Lisinopril and Hydrochlorothiazide Tablets. More marked increases have also been reported and were more likely to occur in patients with renal artery stenosis (see PRECAUTIONS).
Serum Uric Acid, Glucose, Magnesium, Cholesterol, Triglycerides and Calcium: See PRECAUTIONS.
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.5 g percent and 1.5 vol percent, respectively) occurred frequently in hypertensive patients treated with Lisinopril and Hydrochlorothiazide Tablets but were rarely of clinical importance unless another cause of anemia coexisted. In clinical trials, 0.4 percent of patients discontinued therapy due to anemia.
Liver Function Tests: Rarely, elevations of liver enzymes and/or serum bilirubin have occurred (see WARNINGS, Hepatic Failure).
Other adverse reactions that have been reported with the individual components are listed below:
Lisinopril — In clinical trials adverse reactions which occurred with lisinopril were also seen with Lisinopril and Hydrochlorothiazide Tablets. In addition, and since lisinopril has been marketed, the following adverse reactions have been reported with lisinopril and should be considered potential adverse reactions for Lisinopril and Hydrochlorothiazide Tablets: Body as a Whole: Anaphylactoid reactions (see WARNINGS, Anaphylactoid and Possibly Related Reactions), malaise, edema, facial edema, pain, pelvic pain, flank pain, chills; Cardiovascular: Cardiac arrest, myocardial infarction or cerebrovascular accident, possibly secondary to excessive hypotension in high- risk patients (see WARNINGS, Hypotension), pulmonary embolism and infarction, worsening of heart failure, arrhythmias (including tachycardia, ventricular tachycardia, atrial tachycardia, atrial fibrillation, bradycardia, and premature ventricular contractions), angina pectoris, transient ischemic attacks, paroxysmal nocturnal dyspnea, decreased blood pressure, peripheral edema, vasculitis; Digestive: Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) (see WARNINGS, Hepatic Failure), gastritis, anorexia, flatulence, increased salivation; Endocrine: Diabetes mellitus, syndrome of inappropriate antidiuretic hormone secretion (SIADH); Hematologic: Rare cases of neutropenia, thrombocytopenia, and bone marrow depression have been reported. Hemolytic anemia has been reported; a causal relationship to lisinopril cannot be excluded; Metabolic: Gout, weight loss, dehydration, fluid overload, weight gain; Musculoskeletal: Arthritis, arthralgia, neck pain, hip pain, joint pain, leg pain, arm pain, lumbago; Nervous System/Psychiatric: Ataxia, memory impairment, tremor, insomnia, stroke, nervousness, confusion, peripheral neuropathy (e.g., paresthesia, dysesthesia), spasm, hypersomnia, irritability, mood alterations (including depressive symptoms); hallucinations ; Respiratory: Malignant lung neoplasms, hemoptysis, pulmonary edema, pulmonary infiltrates, eosinophilic pneumonitis, bronchospasm, asthma, pleural effusion, pneumonia, wheezing, orthopnea, painful respiration, epistaxis, laryngitis, sinusitis, pharyngitis, rhinitis, rhinorrhea, chest sound abnormalities; Skin: Urticaria, alopecia, herpes zoster, photosensitivity, skin lesions, skin infections, pemphigus, erythema, psoriasis. Other severe skin reactions (including toxic epidermal necrolysis, Stevens-Johnson syndrome and cutaneous pseudolymphoma) have been reported rarely; causal relationship has not been established; Special Senses: Visual loss, diplopia, photophobia, taste disturbances, olfactory disturbances; Urogenital: Acute renal failure, oliguria, anuria, uremia, progressive azotemia, renal dysfunction (see PRECAUTIONS and DOSAGE AND ADMINISTRATION), pyelonephritis, dysuria, breast pain.
Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, leukocytosis, eosinophilia, photosensitivity, rash, and other dermatological manifestations.
Fetal/Neonatal Morbidity and Mortality: See WARNINGS, Pregnancy, Lisinopril, Fetal/Neonatal Morbidity and Mortality.
Hydrochlorothiazide — Body as a Whole: Weakness; Digestive: Anorexia, gastric irritation, cramping, jaundice (intrahepatic cholestatic jaundice) (see WARNINGS, Hepatic Failure), pancreatitis, sialadenitis, constipation; Hematologic: Leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia, hemolyticanemia; Musculoskeletal: Muscle spasm; Nervous System/Psychiatric: Restlessness; Renal: Renal failure, renal dysfunction, interstitial nephritis (see WARNINGS); Skin: Erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis, alopecia; Special Senses: Xanthopsia; Hypersensitivity: Purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions.
Postmarketing Experience
Non-melanoma Skin Cancer
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
To report SUSPECTED ADVERSE REACTIONS, contact Actavis at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Close -
OVERDOSAGE
No specific information is available on the treatment of overdosage with Lisinopril and Hydrochlorothiazide Tablets. Treatment is symptomatic and supportive. Therapy with Lisinopril ...
No specific information is available on the treatment of overdosage with Lisinopril and Hydrochlorothiazide Tablets. Treatment is symptomatic and supportive. Therapy with Lisinopril and Hydrochlorothiazide Tablets should be discontinued and the patient observed closely. Suggested measures include induction of emesis and/or gastric lavage, and correction of dehydration, electrolyte imbalance and hypotension by established procedures.
Lisinopril
Following a single oral dose of 20 g/kg, no lethality occurred in rats and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis (see WARNINGS, Anaphylactoid reactions during membrane exposure).
Hydrochlorothiazide
Oral administration of a single oral dose of 10 g/kg to mice and rats was not lethal. The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
Close -
DOSAGE AND ADMINISTRATION
Lisinopril monotherapy is an effective treatment of hypertension in once-daily doses of 10 mg to 80 mg, while hydrochlorothiazide monotherapy is effective in doses of 12.5 mg to 50 mg per day. In ...
Lisinopril monotherapy is an effective treatment of hypertension in once-daily doses of 10 mg to 80 mg, while hydrochlorothiazide monotherapy is effective in doses of 12.5 mg to 50 mg per day. In clinical trials of lisinopril/hydrochlorothiazide combination therapy using lisinopril doses of 10 mg to 80 mg and hydrochlorothiazide doses of 6.25 mg to 50 mg, the antihypertensive response rates generally increased with increasing dose of either component.
The side effects (see WARNINGS) of lisinopril are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent phenomena (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. Therapy with any combination of lisinopril and hydrochlorothiazide will be associated with both sets of dose-independent side effects, but addition of lisinopril in clinical trials blunted the hypokalemia normally seen with diuretics.
To minimize dose-independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.
Dose Titration Guided by Clinical Effect
A patient whose blood pressure is not adequately controlled with either lisinopril or hydrochlorothiazide monotherapy may be switched to Lisinopril and Hydrochlorothiazide Tablets 10/12.5 or Lisinopril and Hydrochlorothiazide Tablets 20/12.5. Further increases of either or both components could depend on clinical response. The hydrochlorothiazide dose should generally not be increased until 2 to 3 weeks have elapsed. Patients whose blood pressures are adequately controlled with 25 mg of daily hydrochlorothiazide, but who experience significant potassium loss with this regimen, may achieve similar or greater blood pressure control with less potassium loss if they are switched to Lisinopril and Hydrochlorothiazide Tablets 10/12.5. Dosage higher than lisinopril 80 mg and hydrochlorothiazide 50 mg should not be used.
Replacement Therapy
The combination may be substituted for the titrated individual components.
Use in Renal Impairment
The usual regimens of therapy with Lisinopril and Hydrochlorothiazide Tablets need not be adjusted as long as the patient's creatinine clearance is greater than 30 mL/min/1.73 m2 (serum creatinine approximately less than or equal to 3 mg/dL or 265 µmol/L). In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so Lisinopril and Hydrochlorothiazide Tablets is not recommended (see WARNINGS, Anaphylactoid reactions during membrane exposure).
Close -
HOW SUPPLIED
Lisinopril and hydrochlorothiazide tablets USP are available as follows: 10 mg/12.5 mg: Pink, round, unscored, flat-faced, beveled-edge tablets, debossed “WATSON” and “860” on the periphery of one ...
Lisinopril and hydrochlorothiazide tablets USP are available as follows:
10 mg/12.5 mg: Pink, round, unscored, flat-faced, beveled-edge tablets, debossed “WATSON” and “860” on the periphery of one side and plain on the other side are supplied in bottles of 100 (NDC 0591-0860-01) and 500 (NDC 0591-0860-05).
20 mg/12.5 mg: Light blue, round, unscored, flat-faced, beveled-edge tablets, debossed “WATSON” and “861” on the periphery of one side and plain on the other side are supplied in bottles of 100 (NDC 0591-0861-01) and
500 (NDC 0591-0861-05).20 mg/25 mg: Pink, round, unscored, flat-faced, beveled-edge tablets, debossed “WATSON” and “862” on the periphery of one side and plain on the other side are supplied in bottles of 100 (NDC 0591-0862-01) and
500 (NDC 0591-0862-05).Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from excessive light and humidity.
Dispense in a tight, light-resistant container according to USP/NF.
Brands listed are trademarks of their respective owners.
Manufactured in India by:
Watson Pharma Private Limited
Verna, Salcette Goa 403 722 INDIADistributed by:
Actavis Pharma, Inc.
Parsippany, NJ 07054 USARev. B 5/2020
Close -
PRINCIPAL DISPLAY PANEL0591-0860-01 - Lisinopril and - Hydrochlorothiazide - Tablets, USP - 10 mg/12.5 mg - 100 Tablets Rx Only
-
PRINCIPAL DISPLAY PANEL0591-0861-01 - Lisinopril and - Hydrochlorothiazide - Tablets, USP - 20 mg/12.5 mg - 100 Tablets Rx Only
-
PRINCIPAL DISPLAY PANEL0591-0862-01 - Lisinopril and - Hydrochlorothiazide - Tablets, USP - 20 mg/25 mg - 100 Tablets Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information
LISINOPRIL AND HYDROCHLOROTHIAZIDE lisinopril and hydrochlorothiazide tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-0860 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISINOPRIL (UNII: E7199S1YWR) (LISINOPRIL ANHYDROUS - UNII:7Q3P4BS2FD) LISINOPRIL 10 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink Score no score Shape ROUND Size 8mm Flavor Imprint Code WATSON;860 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-0860-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/01/2002 2 NDC:0591-0860-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/01/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076194 07/01/2002 LISINOPRIL AND HYDROCHLOROTHIAZIDE lisinopril and hydrochlorothiazide tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-0861 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISINOPRIL (UNII: E7199S1YWR) (LISINOPRIL ANHYDROUS - UNII:7Q3P4BS2FD) LISINOPRIL 20 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 2--ALUMINUM LAKE (UNII: 4AQJ3LG584) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color blue (Light blue) Score no score Shape ROUND Size 10mm Flavor Imprint Code WATSON;861 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-0861-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/04/2003 2 NDC:0591-0861-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/04/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076194 03/04/2003 LISINOPRIL AND HYDROCHLOROTHIAZIDE lisinopril and hydrochlorothiazide tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-0862 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISINOPRIL (UNII: E7199S1YWR) (LISINOPRIL ANHYDROUS - UNII:7Q3P4BS2FD) LISINOPRIL 20 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink Score no score Shape ROUND Size 10mm Flavor Imprint Code WATSON;862 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-0862-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/04/2003 2 NDC:0591-0862-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/04/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076194 03/04/2003
CloseLabeler - Actavis Pharma, Inc. (119723554)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LISINOPRIL AND HYDROCHLOROTHIAZIDE tablet
Number of versions: 14
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Sep 22, 2022 | 28 (current) | download |
| Jul 12, 2021 | 27 | download |
| Aug 4, 2020 | 26 | download |
| May 28, 2020 | 25 | download |
| May 15, 2020 | 24 | download |
| Nov 28, 2018 | 22 | download |
| Nov 8, 2017 | 21 | download |
| Jan 17, 2017 | 20 | download |
| Feb 8, 2013 | 15 | download |
| Nov 9, 2012 | 12 | download |
| Jul 24, 2012 | 9 | download |
| Jun 25, 2010 | 4 | download |
| May 22, 2008 | 2 | download |
| May 17, 2007 | 1 | download |
RxNorm
LISINOPRIL AND HYDROCHLOROTHIAZIDE tablet
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 197885 | lisinopril 10 MG / hydroCHLOROthiazide 12.5 MG Oral Tablet | PSN |
| 2 | 197885 | hydrochlorothiazide 12.5 MG / lisinopril 10 MG Oral Tablet | SCD |
| 3 | 197885 | HCTZ 12.5 MG / lisinopril 10 MG Oral Tablet | SY |
| 4 | 197886 | lisinopril 20 MG / hydroCHLOROthiazide 12.5 MG Oral Tablet | PSN |
| 5 | 197886 | hydrochlorothiazide 12.5 MG / lisinopril 20 MG Oral Tablet | SCD |
| 6 | 197886 | HCTZ 12.5 MG / lisinopril 20 MG Oral Tablet | SY |
| 7 | 197887 | lisinopril 20 MG / hydroCHLOROthiazide 25 MG Oral Tablet | PSN |
| 8 | 197887 | hydrochlorothiazide 25 MG / lisinopril 20 MG Oral Tablet | SCD |
| 9 | 197887 | HCTZ 25 MG / lisinopril 20 MG Oral Tablet | SY |
Get Label RSS Feed for this Drug
LISINOPRIL AND HYDROCHLOROTHIAZIDE tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=ab5e9ae4-e575-4a63-99f3-2bde36bdd508
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LISINOPRIL AND HYDROCHLOROTHIAZIDE tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0591-0860-01 |
| 2 | 0591-0860-05 |
| 3 | 0591-0861-01 |
| 4 | 0591-0861-05 |
| 5 | 0591-0862-01 |
| 6 | 0591-0862-05 |