Label: LABETALOL HYDROCHLORIDE tablet, film coated
- NDC Code(s): 0591-0605-01, 0591-0605-05, 0591-0605-10, 0591-0606-01, view more
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 31, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
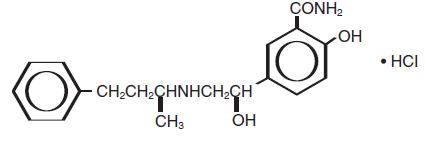
DESCRIPTIONLabetalol hydrochloride (HCl) is an adrenergic receptor blocking agent that has both selective alpha1-adrenergic and nonselective beta-adrenergic receptor blocking actions in a single ...
-
CLINICAL PHARMACOLOGYLabetalol HCl combines both selective, competitive, alpha1-adrenergic blocking and nonselective, competitive, beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha ...
-
INDICATIONS AND USAGELabetalol hydrochloride tablets, USP are indicated in the management of hypertension. Labetalol hydrochloride tablets, USP may be used alone or in combination with other antihypertensive agents ...
-
CONTRAINDICATIONSLabetalol HCl tablets are contraindicated in bronchial asthma, overt cardiac failure, greater-than-first-degree heart block, cardiogenic shock, severe bradycardia, other conditions associated with ...
-
WARNINGSHepatic Injury: Severe hepatocellular injury, confirmed by rechallenge in at least one case, occurs rarely with labetalol therapy. The hepatic injury is usually reversible, but hepatic necrosis ...
-
PRECAUTIONSGeneral: Impaired Hepatic Function: Labetalol HCl tablets should be used with caution in patients with impaired hepatic function since metabolism of the drug may be diminished. Intraoperative ...
-
ADVERSE REACTIONSMost adverse effects are mild and transient and occur early in the course of treatment. In controlled clinical trials of 3 to 4 months’ duration, discontinuation of labetalol HCl tablets due to ...
-
OVERDOSAGEOverdosage with labetalol HCl causes excessive hypotension that is posture sensitive and, sometimes, excessive bradycardia. Patients should be placed supine and their legs raised if necessary to ...
-
DOSAGE AND ADMINISTRATIONDOSAGE MUST BE INDIVIDUALIZED. The recommended initial dosage is 100 mg twice daily whether used alone or added to a diuretic regimen. After 2 or 3 days, using standing blood pressure as an ...
-
HOW SUPPLIEDLabetalol HCl Tablets, USP 100 mg are available as round, beige, film-coated tablets debossed with “WATSON 605” on one side and scored on the other side. They are supplied in bottles of 100, 500 ...
-
PRINCIPAL DISPLAY PANELNDC 0591-0605-01 - Labetalol - Hydrochloride - Tablets, USP - 100 mg - 100 Tablets - Rx only
-
PRINCIPAL DISPLAY PANELNDC 0591-0606-01 - Labetalol - Hydrochloride - Tablets, USP - 200 mg - 100 Tablets Rx only
-
PRINCIPAL DISPLAY PANELNDC 0591-0607-01 - Labetalol - Hydrochloride - Tablets, USP - 300 mg - 100 Tablets Rx only
-
INGREDIENTS AND APPEARANCEProduct Information