Label: TRETINOIN gel
TRETINOIN cream
- NDC Code(s): 0574-2201-20, 0574-2201-45, 0574-2205-20, 0574-2205-45, view more
- Packager: Padagis US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor External Use Only. Not For Ophthalmic Use. Rx Only - Prescribing Information
-
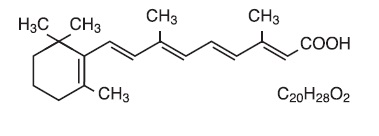
Description:Tretinoin Gel, USP and Tretinoin Cream, USP are used for the topical treatment of acne vulgaris. Each gram of tretinoin gel contains tretinoin in either of two strengths, 0.025% (0.25 mg) or 0.01 ...
-
Clinical Pharmacology: Although the exact mode of action of tretinoin is unknown, current evidence suggests that topical tretinoin decreases cohesiveness of follicular epithelial cells with decreased microcomedo ...
-
Indications and Usage: Tretinoin gel and cream are indicated for topical application in the treatment of acne vulgaris. The safety and efficacy of the long-term use of this product in the treatment of other disorders ...
-
Contraindications: Use of the product should be discontinued if hypersensitivity to any of the ingredients is noted.
-
Warnings: GELS ARE FLAMMABLE. AVOID FIRE, FLAME OR SMOKING DURING USE. Keep out of reach of children. Keep tube tightly closed. Do not expose to heat or store at temperatures above 120°F (49°C).
-
Precautions: General: If a reaction suggesting sensitivity or chemical irritation occurs, use of the medication should be discontinued. Exposure to sunlight, including sunlamps, should be minimized ...
-
Adverse Reactions: The skin of certain sensitive individuals may become excessively red, edematous, blistered, or crusted. If these effects occur, the medication should either be discontinued until the integrity of ...
-
Overdosage: If medication is applied excessively, no more rapid or better results will be obtained and marked redness, peeling, or discomfort may occur. Oral ingestion of the drug may lead to the same side ...
-
Dosage and Administration: Tretinoin gel or cream should be applied once a day, before retiring, to the skin where acne lesions appear, using enough to cover the entire affected area lightly. Gel: Excessive application ...
-
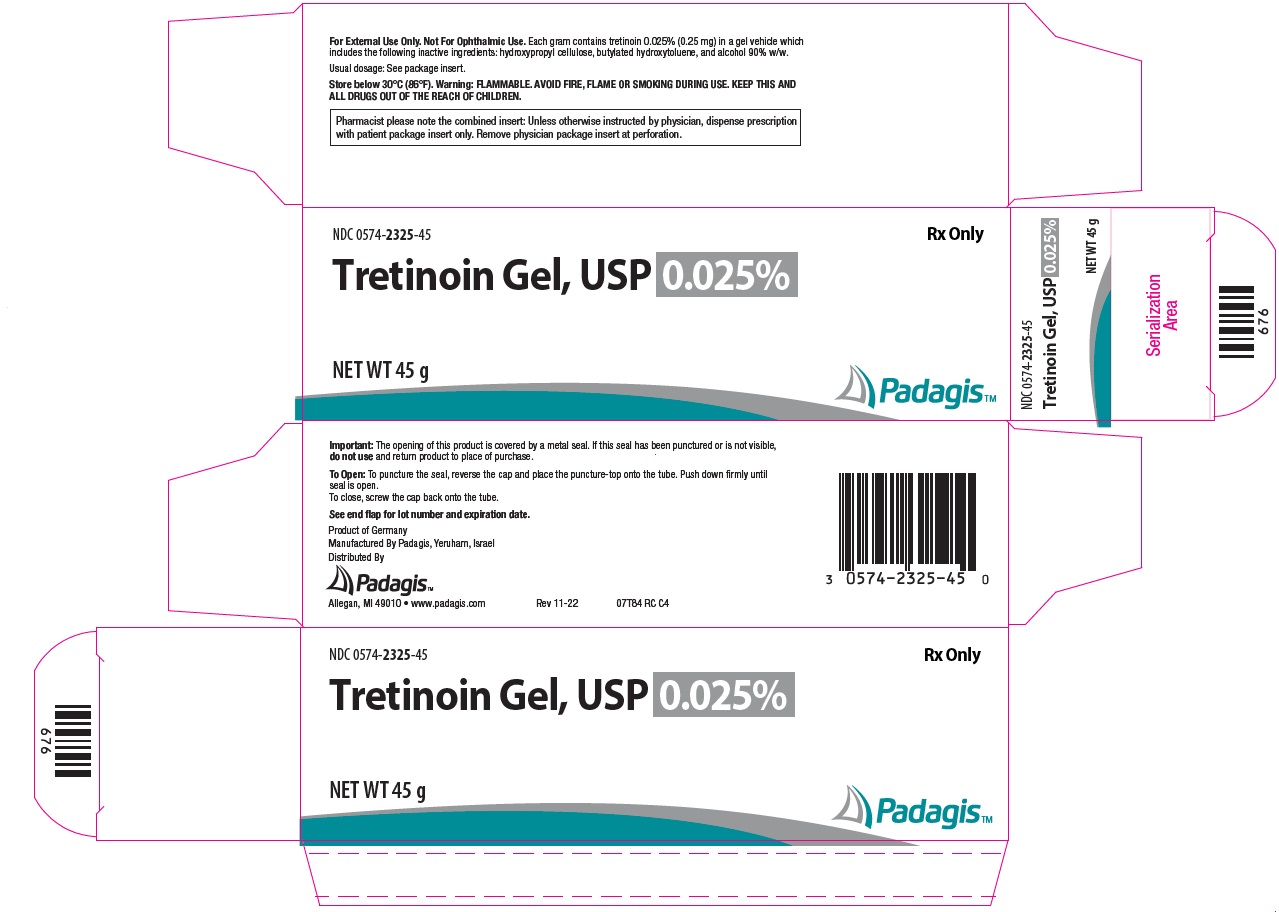
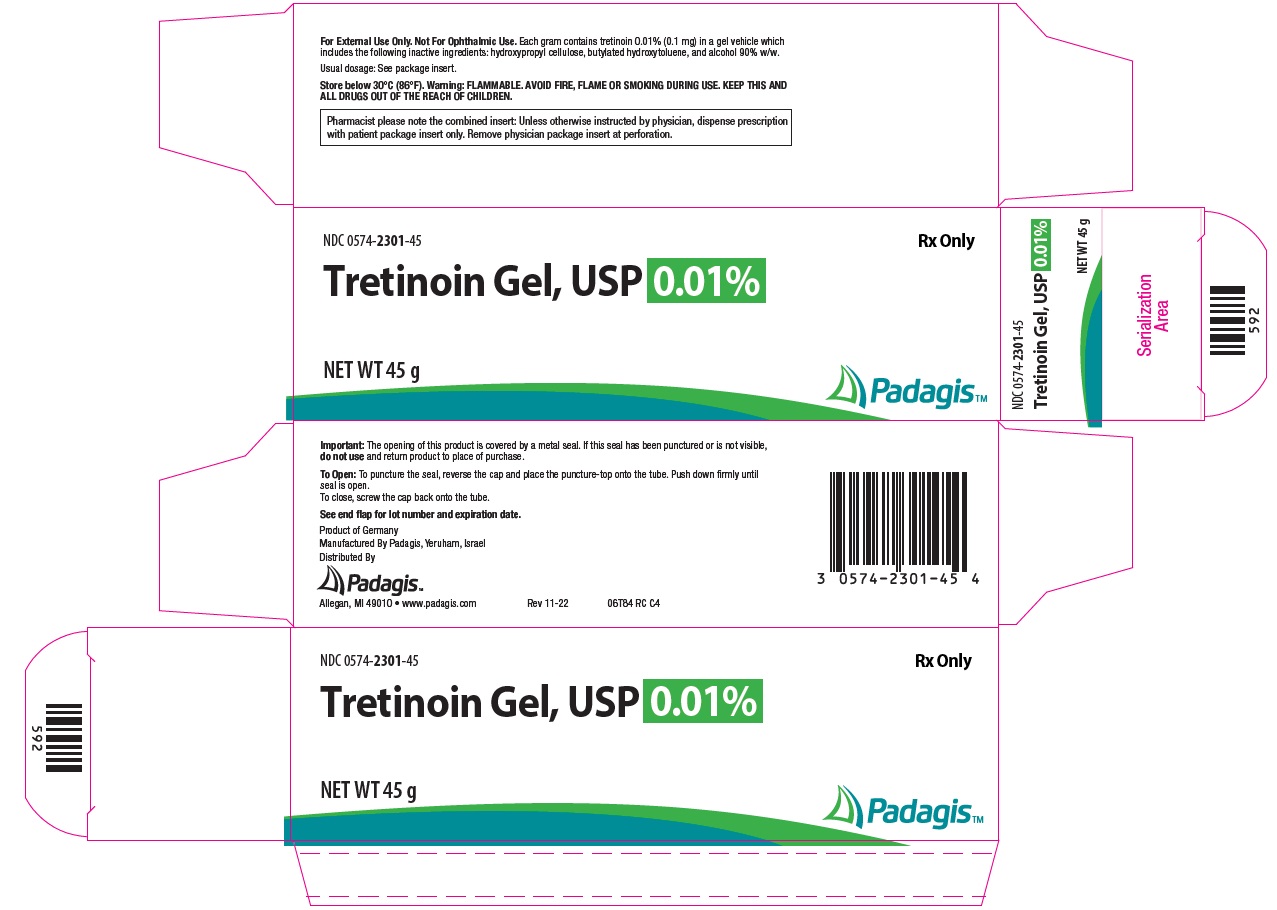
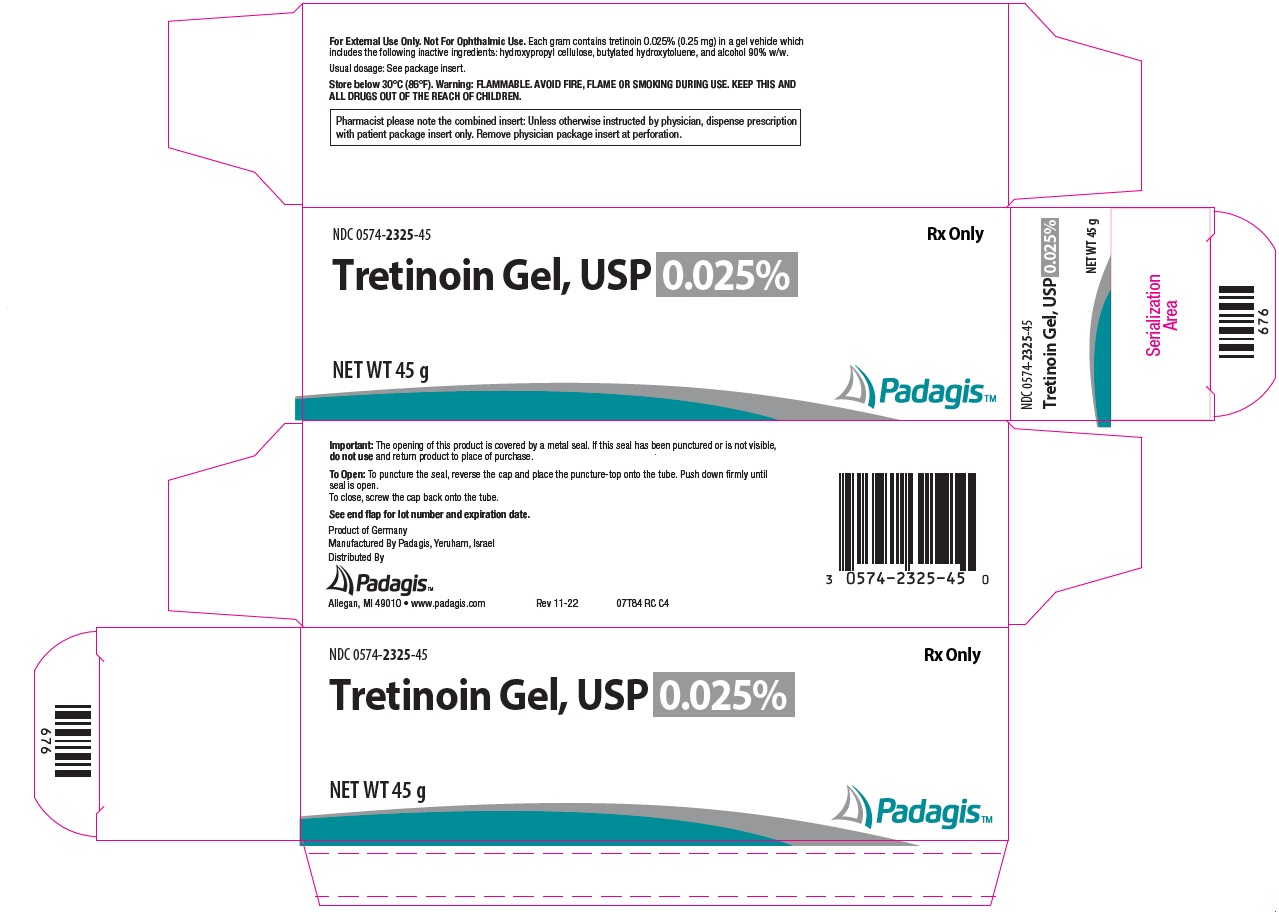
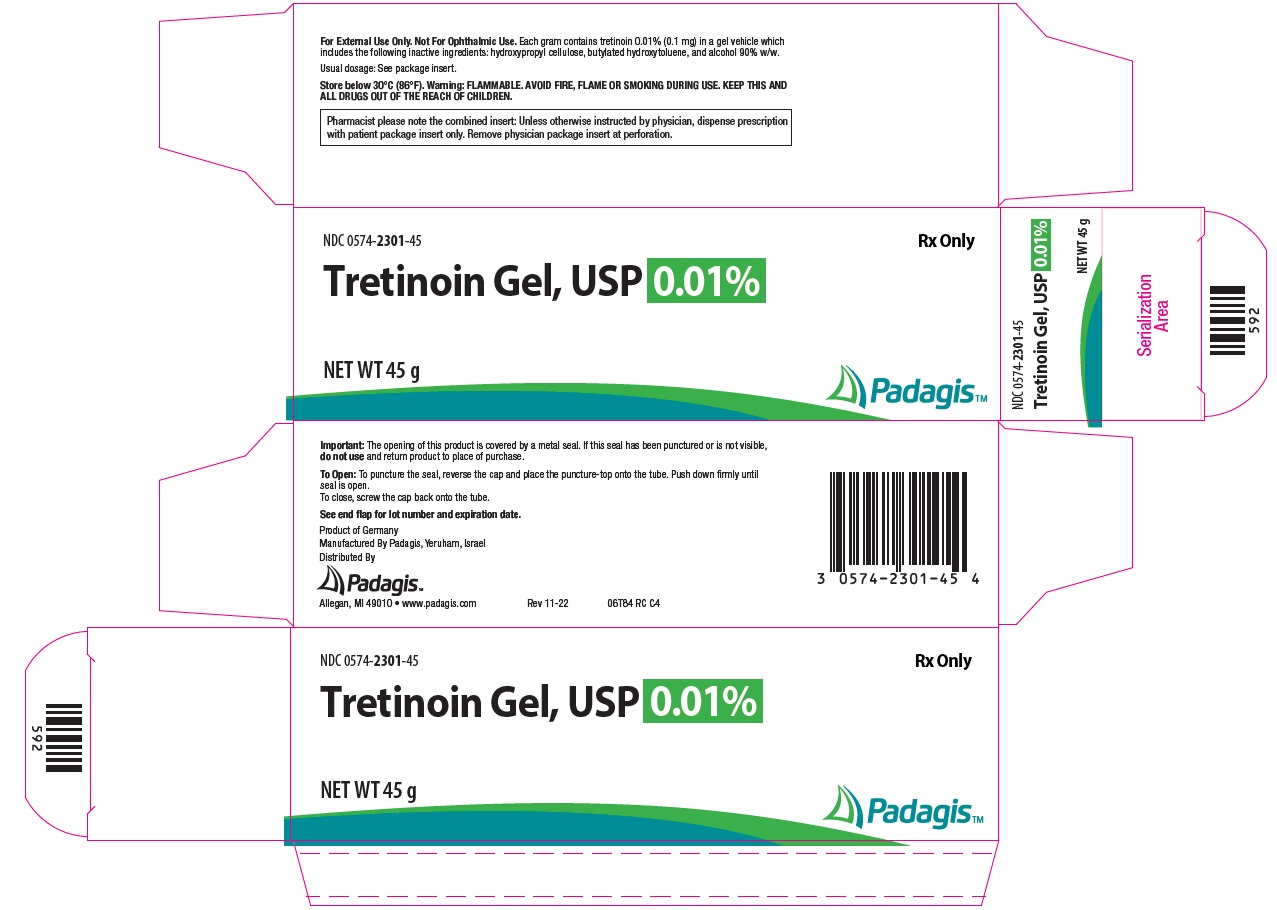
How Supplied: Tretinoin Gel, USP - NDC CODE - Strength - Qty. 0574-2325-15 - 0.025% 15 g - 0574-2325-45 - 0.025% 45 g - 0574-2301-15 - 0.01% 15 g - 0574-2301-45 - 0.01% 45 g - Tretinoin ...
-
Storage Conditions: Tretinoin Gel, USP: store below 30°C (86°F). Tretinoin Cream, USP: store below 27°C (80°F)
-
SPL UNCLASSIFIED SECTIONManufactured by Padagis® Yeruham, Israel - www.padagis.com - Rev 12-23 - 08T00 RC PH7
-
PATIENT INSTRUCTIONS Acne Treatment - IMPORTANT Read Directions Carefully Before Using - THIS LEAFLET TELLS YOU ABOUT TRETINOIN ACNE TREATMENT AS PRESCRIBED BY YOUR PHYSICIAN. THIS PRODUCT IS TO BE USED ONLY ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Gel 0.025% NDC 0574-2325-45 - Rx Only - Tretinoin Gel, USP 0.025% NET WT 45 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package ...
-
Package/Label Display Panel – Gel 0.01% NDC 0574-2301-45 - Rx Only - Tretinoin Gel, USP 0.01% NET WT 45 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package ...
-
Package/Label Display Panel – Cream 0.1% NDC 0574-2201-20 - Rx Only - Tretinoin Cream, USP 0.1% NET WT 20 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package ...
-
Package/Label Display Panel – Cream 0.05% NDC 0574-2205-20 - Rx Only - Tretinoin Cream, USP 0.05% NET WT 20 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package ...
-
Package/Label Display Panel - Cream 0.025% NDC 0574-2225-20 - Rx Only - Tretinoin Cream, USP 0.025% NET WT 20 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package ...
-
INGREDIENTS AND APPEARANCEProduct Information