Label: NYSTOP- nystatin powder
- NDC Code(s): 0574-2008-02, 0574-2008-15, 0574-2008-30
- Packager: Padagis US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
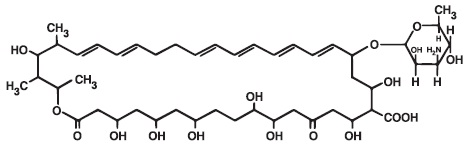
DESCRIPTIONNystatin is a polyene antifungal antibiotic obtained from Streptomyces nursei. The molecular formula for Nystatin is C47H75NO17. The molecular weight of Nystatin is 926.1. Structural ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - Nystatin is not absorbed from intact skin or mucous membrane. Microbiology - Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide ...
-
INDICATIONS AND USAGENystatin Topical Powder is indicated in the treatment of cutaneous or mucocutaneous mycotic infections caused by Candida albicans and other susceptible Candida species. This preparation is not ...
-
CONTRAINDICATIONSNystatin Topical Powder is contraindicated in patients with a history of hypersensitivity to any of its components.
-
PRECAUTIONSGeneral - Nystatin Topical Powder should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections. If irritation or sensitization develops, treatment should be ...
-
ADVERSE REACTIONSThe frequency of adverse events reported in patients using nystatin topical preparations is less than 0.1%. The more common events that were reported include allergic reactions, burning, itching ...
-
DOSAGE AND ADMINISTRATIONVery moist lesions are best treated with the topical dusting powder. Adults and Pediatric Patients (Neonates and Older): Apply to candidal lesions two or three times daily until healing is ...
-
HOW SUPPLIEDNystop® Nystatin Topical Powder USP is supplied as 100,000 units nystatin per gram in 15 g, 30 g and 60 g plastic squeeze bottles. (NDC 0574-2008-15) (NDC 0574-2008-30) (NDC ...
-
PRINCIPAL DISPLAY PANELNDC 0574-2008-15 - Rx Only - NYSTOP® Nystatin Topical Powder, USP - 100,000 USP Units Per Gram - USUAL DOSAGE: Apply to affected area 2 or 3 times daily. STORAGE: Store at controlled room temperature ...
-
INGREDIENTS AND APPEARANCEProduct Information