Label: ESTRADIOL tablet
-
NDC Code(s):
0555-0886-02,
0555-0886-04,
0555-0887-02,
0555-0887-04, view more0555-0899-02
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - ESTROGENS INCREASE THE RISK OF ENDOMETRIAL CANCER - Close clinical surveillance of all women taking estrogens is important. Adequate diagnostic measures, including endometrial ...
Rx only
CloseESTROGENS INCREASE THE RISK OF ENDOMETRIAL CANCER
Close clinical surveillance of all women taking estrogens is important. Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is no evidence that the use of “natural” estrogens results in a different endometrial risk profile than “synthetic” estrogens at equivalent estrogen doses. (See WARNINGS, Malignant neoplasms, Endometrial cancer.)
CARDIOVASCULAR AND OTHER RISKS
Estrogens with or without progestins should not be used for the prevention of cardiovascular disease. (See WARNINGS, Cardiovascular disorders.)
The Women’s Health Initiative (WHI) study reported increased risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and deep vein thrombosis in postmenopausal women (50 to 79 years of age) during 5 years of treatment with oral conjugated estrogens (CE 0.625 mg) combined with medroxyprogesterone acetate (MPA 2.5 mg) relative to placebo. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
The Women’s Health Initiative Memory Study (WHIMS), a substudy of WHI, reported increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with oral conjugated estrogens plus medroxyprogesterone acetate relative to placebo. It is unknown whether this finding applies to younger postmenopausal women or to women taking estrogen alone therapy. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
Other doses of oral conjugated estrogens with medroxyprogesterone acetate, and other combinations and dosage forms of estrogens and progestins were not studied in the WHI clinical trials and, in the absence of comparable data, these risks should be assumed to be similar. Because of these risks, estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
-
DESCRIPTIONEstradiol Tablets, USP for oral administration contains 0.5, 1 or 2 mg of micronized estradiol, USP per tablet. Estradiol, USP (17β-estradiol) is a white, crystalline solid, chemically described ...
Estradiol Tablets, USP for oral administration contains 0.5, 1 or 2 mg of micronized estradiol, USP per tablet. Estradiol, USP (17β-estradiol) is a white, crystalline solid, chemically described as estra-1,3,5,(10)-triene-3, 17β-diol. The structural formula is:
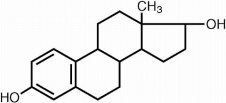
C18H24O2 M.W. 272.38
Inactive Ingredients: Colloidal silicon dioxide, corn starch, dibasic calcium phosphate, lactose monohydrate, magnesium stearate, and sodium starch glycolate. In addition, the 1 mg also contains FD&C blue no. 1 aluminum lake and D&C red no. 27 aluminum lake. The 2 mg also contains FD&C blue no. 1 aluminum lake and FD&C yellow no. 5 (tartrazine) aluminum lake.
Close -
CLINICAL PHARMACOLOGYEndogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a ...
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
Pharmacokinetics
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to sex hormone binding globulin (SHBG) and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Special Populations
No pharmacokinetic studies were conducted in special populations, including patients with renal or hepatic impairment.
Drug Interactions
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4 such as St. John’s Wort preparations (Hypericum perforatum), phenobarbital, carbamazepine, and rifampin may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may increase plasma concentrations of estrogens and may result in side effects.
CloseClinical Studies
Osteoporosis
Most prospective studies of efficacy for this indication have been carried out in white menopausal women, without stratification by other risk factors, and tend to show a universally salutary effect on bone.
The results of a two-year, randomized, placebo-controlled, double-blind, dose-ranging study have shown that treatment with 0.5 mg estradiol daily for 23 days (of a 28 day cycle) prevents vertebral bone mass loss in postmenopausal women. When estrogen therapy is discontinued, bone mass declines at a rate comparable to the immediate postmenopausal period. There is no evidence that estrogen replacement therapy restores bone mass to premenopausal levels.
Women’s Health Initiative Studies
The Women’s Health Initiative (WHI) enrolled a total of 27,000 predominantly healthy postmenopausal women to assess the risks and benefits of either the use of oral 0.625 mg conjugated estrogens (CE) per day alone or the use of oral 0.625 mg conjugated estrogens plus 2.5 mg medroxyprogesterone acetate (MPA) per day compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) (nonfatal myocardial infarction and CHD death), with invasive breast cancer as the primary adverse outcome studied. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, pulmonary embolism (PE), endometrial cancer, colorectal cancer, hip fracture, or death due to other cause. The study did not evaluate the effects of CE or CE/MPA on menopausal symptoms.
The CE/MPA substudy was stopped early because, according to the predefined stopping rule, the increased risk of breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” Results of the CE/MPA substudy, which included 16,608 women (average age of 63 years, range 50 to 79; 83.9% White, 6.5% Black, 5.5% Hispanic), after an average follow-up of 5.2 years are presented in Table 1 below:
Table 1: RELATIVE AND ABSOLUTE RISK SEEN IN THE CE/MPA SUBSTUDY OF WHI* - *
- adapted from JAMA, 2002; 288:321-333
- †
- a subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes
- ‡
- nominal confidence intervals unadjusted for multiple looks and multiple comparisons
- §
- includes metastatic and non-metastatic breast cancer with the exception of in situ breast cancer
- ¶
- not included in Global Index
Event†
Relative Risk
CE/MPA vs placebo
at 5.2 Years
(95% CI‡)
Placebo
n = 8102
CE/MPA
n = 8506
Absolute Risk per 10,000 Women-Years
CHD events
Non-fatal MI
CHD death
1.29 (1.02 to 1.63)
1.32 (1.02 to 1.72)
1.18 (0.70 to 1.97)
30
23
6
37
30
7
Invasive breast cancer§
1.26 (1.00 to 1.59)
30
38
Stroke
1.41 (1.07 to 1.85)
21
29
Pulmonary embolism
2.13 (1.39 to 3.25)
8
16
Colorectal cancer
0.63 (0.43 to 0.92)
16
10
Endometrial cancer
0.83 (0.47 to 1.47)
6
5
Hip fracture
0.66 (0.45 to 0.98)
15
10
Death due to causes other than the events above
0.92 (0.74 to 1.14)
40
37
Global Index†
1.15 (1.03 to 1.28)
151
170
Deep vein thrombosis¶
2.07 (1.49 to 2.87)
13
26
Vertebral fractures¶
0.66 (0.44 to 0.98)
15
9
Other osteoporotic fractures¶
0.77 (0.69 to 0.86)
170
131
For those outcomes included in the “global index,” the absolute excess risks per 10,000 women-years in the group treated with CE/MPA were 7 more CHD events, 8 more strokes, 8 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures. The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality. (See BOXED WARNINGS, WARNINGS, and PRECAUTIONS.)
Women’s Health Initiative Memory Study
The Women’s Health Initiative Memory Study (WHIMS), a substudy of WHI, enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47% were age 65 to 69 years, 35% were 70 to 74 years, and 18% were 75 years of age and older) to evaluate the effects of CE/MPA (0.625 mg conjugated estrogens plus 2.5 mg medroxyprogesterone acetate) on the incidence of probable dementia (primary outcome) compared with placebo.
After an average follow-up of 4 years, 40 women in the estrogen/progestin group (45 per 10,000 women-years) and 21 in the placebo group (22 per 10,000 women-years) were diagnosed with probable dementia. The relative risk of probable dementia in the hormone therapy group was 2.05 (95% CI, 1.21 to 3.48) compared to placebo. Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See BOXED WARNING and WARNINGS, Dementia.)
-
INDICATIONS AND USAGEEstradiol tablets are indicated in the: Treatment of moderate to severe vasomotor symptoms associated with the menopause. Treatment of moderate to severe symptoms of vulvar and vaginal atrophy ...
Estradiol tablets are indicated in the:
- Treatment of moderate to severe vasomotor symptoms associated with the menopause.
- Treatment of moderate to severe symptoms of vulvar and vaginal atrophy associated with the menopause. When prescribing solely for the treatment of symptoms of vulvar and vaginal atrophy, topical vaginal products should be considered.
- Treatment of hypoestrogenism due to hypogonadism, castration or primary ovarian failure.
- Treatment of breast cancer (for palliation only) in appropriately selected women and men with metastatic disease.
- Treatment of advanced androgen-dependent carcinoma of the prostate (for palliation only).
- Prevention of osteoporosis. When prescribing solely for the prevention of postmenopausal osteoporosis, therapy should only be considered for women at significant risk of osteoporosis and for whom non-estrogen medications are not considered to be appropriate. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
The mainstays for decreasing the risk of postmenopausal osteoporosis are weight bearing exercise, adequate calcium and vitamin D intake, and when indicated, pharmacologic therapy. Postmenopausal women require an average of 1500 mg/day of elemental calcium. Therefore, when not contraindicated, calcium supplementation may be helpful for women with suboptimal dietary intake. Vitamin D supplementation of 400 to 800 IU/day may also be required to ensure adequate daily intake in postmenopausal women.
Close -
CONTRAINDICATIONSEstrogens should not be used in individuals with any of the following conditions: Undiagnosed abnormal genital bleeding. Known, suspected or history of cancer of the breast except in ...
Estrogens should not be used in individuals with any of the following conditions:
- Undiagnosed abnormal genital bleeding.
- Known, suspected or history of cancer of the breast except in appropriately selected patients being treated for metastatic disease.
- Known or suspected estrogen-dependent neoplasia.
- Active deep vein thrombosis, pulmonary embolism or history of these conditions.
- Active or recent (e.g., within the past year) arterial thromboembolic disease (e.g., stroke, myocardial infarction.
- Liver dysfunction or disease.
- Estradiol tablets should not be used in patients with known hypersensitivity to its ingredients. Estradiol tablets 2 mg contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
- Known or suspected pregnancy. There is no indication for estradiol tablets in pregnancy. There appears to be little or no increased risk of birth defects in children born to women who have used estrogens and progestins from oral contraceptives inadvertently during early pregnancy. (See PRECAUTIONS.)
-
WARNINGSSee BOXED WARNINGS. 1. Cardiovascular disorders - Estrogen and estrogen/progestin therapy has been associated with an increased risk of cardiovascular events such as myocardial infarction and ...
See BOXED WARNINGS.
1. Cardiovascular disorders
Estrogen and estrogen/progestin therapy has been associated with an increased risk of cardiovascular events such as myocardial infarction and stroke, as well as venous thrombosis and pulmonary embolism (venous thromboembolism or VTE). Should any of these occur or be suspected estrogens should be discontinued immediately.
Risk factors for arterial vascular disease (e.g., hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (e.g., personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
a. Coronary heart disease and stroke
In the Women’s Health Initiative (WHI) study, an increase in the number of myocardial infarctions and strokes has been observed in women receiving CE compared to placebo. These observations are preliminary, and the study is continuing. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
In the CE/MPA substudy of WHI, an increased risk of coronary heart disease (CHD) events (defined as nonfatal myocardial infarction and CHD death) was observed in women receiving CE/MPA compared to women receiving placebo (37 vs 30 per 10,000 women years). The increase in risk was observed in year one and persisted.
In the same substudy of WHI, an increased risk of stroke was observed in women receiving CE/MPA compared to women receiving placebo (29 vs 21 per 10,000 women-years). The increase in risk was observed after the first year and persisted.
In postmenopausal women with documented heart disease (n = 2,763, average age 66.7 years) a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS) treatment with CE/MPA (0.625 mg/2.5 mg per day) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE/MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE/MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred and twenty one women from the original HERS trial agreed to participate in an open label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE/MPA group and the placebo group in HERS, HERS II, and overall.
Large doses of estrogen (5 mg conjugated estrogens per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men to increase the risks of nonfatal myocardial infarction, pulmonary embolism, and thrombophlebitis.
b. Venous thromboembolism (VTE)
In the Women’s Health Initiative (WHI) study, an increase in VTE has been observed in women receiving CE compared to placebo. These observations are preliminary, and the study is continuing. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
In the CE/MPA substudy of WHI, a 2fold greater rate of VTE, including deep venous thrombosis and pulmonary embolism, was observed in women receiving CE/MPA compared to women receiving placebo. The rate of VTE was 34 per 10,000 womenyears in the CE/MPA group compared to 16 per 10,000 womenyears in the placebo group. The increase in VTE risk was observed during the first year and persisted.
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
2. Malignant neoplasms
a. Endometrial cancer
The use of unopposed estrogens in women with intact uteri has been associated with an increased risk of endometrial cancer. The reported endometrial cancer risk among unopposed estrogen users is about 2- to 12- fold greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogens for less than one year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for five to ten years or more and this risk persists for 8 to over 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women taking estrogen/progestin combinations is important (see PRECAUTIONS). Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
b. Breast cancer
The most important randomized clinical trial providing information about breast cancer in estrogen plus progestin users is the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg). After a mean follow-up of 5.6 years, the estrogen plus progestin substudy reported an increased risk of invasive breast cancer in women who took daily CE plus MPA. In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years, for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare, with no apparent difference between the two groups. Other prognostic factors, such as histologic subtype, grade and hormone receptor status did not differ between the groups.
The most important randomized clinical trial providing information about breast cancer in estrogen-alone users is the WHI substudy of daily CE (0.625 mg)-alone. In the WHI estrogen-alone substudy, after an average follow-up of 7.1 years, daily CE (0.625 mg)-alone was not associated with an increased risk of invasive breast cancer [relative risk (RR) 0.80].
Consistent with the Women’s Health Initiative (WHI clinical trials), observational studies have also reported an increased risk of breast cancer for estrogen plus progestin therapy and a smaller, but still increased risk, for estrogen-alone therapy after several years of use. One large meta-analysis of prospective cohort studies reported increased risks that were dependent upon duration of use and could last up to >10 years after discontinuation of estrogen plus progestin therapy and estrogen-alone therapy. Extension of the WHI trials also demonstrated increased breast cancer risk associated with estrogen plus progestin therapy. Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to the risk with estrogen-alone therapy. However, these studies have not found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation. All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors and prior mammogram results.
c. Ovarian cancer
The WHI estrogen plus progestin substudy reported a statistically non-significant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI, 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years] vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27 to 1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
3. Dementia
In the Women’s Health Initiative Memory Study (WHIMS), 4,532 generally healthy postmenopausal women 65 years of age and older were studied, of whom 35% were 70 to 74 years of age and 18% were 75 or older. After an average follow-up of 4 years, 40 women being treated with CE/MPA (1.8%, n = 2,229) and 21 women in the placebo group (0.9%, n = 2,303) received diagnoses of probable dementia. The relative risk for CE/MPA versus placebo was 2.05 (95% confidence interval 1.21 to 3.48), and was similar for women with and without histories of menopausal hormone use before WHIMS. The absolute risk of probable dementia for CE/MPA versus placebo was 45 versus 22 cases per 10,000 women-years, and the absolute excess risk for CE/MPA was 23 cases per 10,000 women-years. It is unknown whether these findings apply to younger postmenopausal women. (See CLINICAL PHARMACOLOGY, Clinical Studies and PRECAUTIONS, Geriatric Use.)
It is unknown whether these findings apply to estrogen alone therapy.
4. Gallbladder disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
5. Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
6. Visual abnormalities
Retinal vascular thrombosis has been reported in patients receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Close -
PRECAUTIONSThis product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C ...
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
A. General
1. Addition of a progestin when a woman has not had a hysterectomy
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks which may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include a possible increased risk of breast cancer.
2. Elevated blood pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogens on blood pressure was not seen. Blood pressure should be monitored at regular intervals with estrogen use.
3. Hypertriglyceridemia
In patients with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis and other complications.
4. Impaired liver function and past history of cholestatic jaundice
Estrogens may be poorly metabolized in patients with impaired liver function. For patients with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised and in the case of recurrence, medication should be discontinued.
5. Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Patients with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Patients dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. These patients should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
6. Fluid retention
Because estrogens may cause some degree of fluid retention, patients with conditions that might be influenced by this factor, such as asthma, epilepsy, migraine, and cardiac or renal dysfunction, warrant careful observation when estrogens are prescribed.
8. Exacerbation of endometriosis
Endometriosis may be exacerbated with administration of estrogens. A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen alone therapy. For patients known to have residual endometriosis post-hysterectomy, the addition of progestin should be considered.
9. Exacerbation of other conditions
Estrogens may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine or porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions.
Estradiol tablets 2 mg contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
B. Patient Information
Physicians are advised to discuss the PATIENT INFORMATION leaflet with patients for whom they prescribe estradiol tablets.
C. Laboratory Tests
Estrogen administration should be initiated at the lowest dose approved for the indication and then guided by clinical response rather than by serum hormone levels (e.g., estradiol, FSH). (See DOSAGE AND ADMINISTRATION section.)
D. Drug/Laboratory Test Interactions
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- Increased thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Patients on thyroid replacement therapy may require higher doses of thyroid hormone.
- Other binding proteins may be elevated in serum, i.e., corticosteroid binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased circulating corticosteroids and sex steroids, respectively. Free hormone concentrations may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
- Increased plasma HDL and HDL2 subfraction concentrations, reduced LDL cholesterol concentration, increased triglycerides levels.
- Impaired glucose tolerance.
- Reduced response to metyrapone test.
E. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term continuous administration of estrogen, with and without progestin, in women with and without a uterus, has shown an increased risk of endometrial cancer, breast cancer, and ovarian cancer. (See BOXED WARNINGS, WARNINGS and PRECAUTIONS.)
Long term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
G. Nursing Mothers
Estrogen administration to nursing mothers has been shown to decrease the quantity and quality of the milk. Detectable amounts of estrogens have been identified in the milk of mothers receiving this drug. Caution should be exercised when estradiol is administered to a nursing woman.
H. Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Large and repeated doses of estrogen over an extended period of time have been shown to accelerate epiphyseal closure, resulting in short adult stature if treatment is initiated before the completion of physiologic puberty in normally developing children. In patients in whom bone growth is not complete, periodic monitoring of bone maturation and effects on epiphyseal centers is recommended.
Estrogen treatment of prepubertal children also induces premature breast development and vaginal cornification, and may potentially induce vaginal bleeding in girls. In boys, estrogen treatment may modify the normal pubertal process. All other physiological and adverse reactions shown to be associated with estrogen treatment of adults could potentially occur in the pediatric population, including thromboembolic disorders and growth stimulation of certain tumors. Therefore, estrogens should only be administered to pediatric patients when clearly indicated and the lowest effective dose should always be utilized.
CloseI. Geriatric Use
The safety and efficacy of estradiol tablets in geriatric patients has not been established. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greatest frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
In the Women’s Health Initiative Memory Study, including 4,532 women 65 years of age and older, followed for an average of 4 years, 82% (n = 3,729) were 65 to 74 while 18% (n = 803) were 75 and over. Most women (80%) had no prior hormone therapy use. Women treated with conjugated estrogens plus medroxyprogesterone acetate were reported to have a two-fold increase in the risk of developing probable dementia. Alzheimer’s disease was the most common classification of probable dementia in both the conjugated estrogens plus medroxyprogesterone acetate group and the placebo group. Ninety percent of the cases of probable dementia occurred in the 54% of women that were older than 70. (See WARNINGS, Dementia.)
It is unknown whether these findings apply to estrogen alone therapy.
-
ADVERSE REACTIONSSee BOXED WARNINGS, WARNINGS, and PRECAUTIONS. The following additional adverse reactions have been reported with estrogen and/or progestin therapy. 1. Genitourinary system - Changes in vaginal ...
See BOXED WARNINGS, WARNINGS, and PRECAUTIONS.
The following additional adverse reactions have been reported with estrogen and/or progestin therapy.
1. Genitourinary system
Changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow; breakthrough bleeding, spotting, dysmenorrhea
Increase in size of uterine leiomyomata
Vaginitis, including vaginal candidiasis
Change in amount of cervical secretion
Changes in cervical ectropion
Ovarian cancer; endometrial hyperplasia; endometrial cancer
2. Breasts
Tenderness, enlargement, pain, nipple discharge, galactorrhea; fibrocystic breast changes; breast cancer
3. Cardiovascular
Deep and superficial venous thrombosis; pulmonary embolism; thrombophlebitis; myocardial infarction; stroke; increase in blood pressure
4. Gastrointestinal
Nausea, vomiting
Abdominal cramps, bloating
Cholestatic jaundice
Increased incidence of gallbladder disease
Pancreatitis
Enlargement of hepatic hemangiomas
5. Skin
Chloasma or melasma that may persist when drug is discontinued
Erythema multiforme
Erythema nodosum
Hemorrhagic eruption
Loss of scalp hair
Hirsutism
Pruritus, rash
7. Central Nervous System
Headache, migraine, dizziness
Mental depression
Chorea
Nervousness, mood disturbances, irritability
Exacerbation of epilepsy
Dementia
Close8. Miscellaneous
Increase or decrease in weight
Reduced carbohydrate tolerance
Aggravation of porphyria
Edema
Arthralgias; leg cramps
Changes in libido
Urticaria
Angioedema
Anaphylactoid/anaphylactic reactions
Hypocalcemia
Exacerbation of asthma
Increased triglycerides
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch.
-
OVERDOSAGESerious ill effects have not been reported following acute ingestion of large doses of estrogen-containing oral contraceptives by young children. Overdosage of estrogen may cause nausea and ...
Serious ill effects have not been reported following acute ingestion of large doses of estrogen-containing oral contraceptives by young children. Overdosage of estrogen may cause nausea and vomiting, and withdrawal bleeding may occur in females.
Close -
DOSAGE AND ADMINISTRATIONWhen estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need ...
When estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need progestin. Use of estrogen, alone or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Patients should be reevaluated periodically as clinically appropriate (e.g., 3-month to 6-month intervals) to determine if treatment is still necessary (see BOXED WARNINGS and WARNINGS). For women who have a uterus, adequate diagnostic measures, such as endometrial sampling, when indicated, should be undertaken to rule out malignancy in cases of undiagnosed persistent or recurring abnormal vaginal bleeding.
Patients should be started at the lowest dose for the indication.
1. For treatment of moderate to severe vasomotor symptoms, vulval and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible.
Attempts to discontinue or taper medication should be made at 3-month to 6-month intervals. The usual initial dosage range is 1 to 2 mg daily of estradiol adjusted as necessary to control presenting symptoms. The minimal effective dose for maintenance therapy should be determined by titration. Administration should be cyclic (e.g., 3 weeks on and 1 week off).
2. For treatment of female hypoestrogenism due to hypogonadism, castration, or primary ovarian failure.
Treatment is usually initiated with a dose of 1 to 2 mg daily of estradiol, adjusted as necessary to control presenting symptoms; the minimal effective dose for maintenance therapy should be determined by titration.
3. For treatment of breast cancer, for palliation only, in appropriately selected women and men with metastatic disease.
Suggested dosage is 10 mg three times daily for a period of at least three months.
4. For treatment of advanced androgen-dependent carcinoma of the prostate, for palliation only.
Suggested dosage is 1 to 2 mg three times daily. The effectiveness of therapy can be judged by phosphatase determinations as well as by symptomatic improvement of the patient.
5. For prevention of osteoporosis.
When prescribing solely for the prevention of postmenopausal osteoporosis, therapy should be considered only for women at significant risk of osteoporosis and for whom non-estrogen medications are not considered to be appropriate.
The lowest effective dose of estradiol has not been determined.
Close -
HOW SUPPLIEDEstradiol Tablets, USP are available as: 0.5 mg: White to off-white, oval, flat-faced, beveled-edge, scored tablet. Debossed with 899 / 1/2 on the scored side and ...
Estradiol Tablets, USP are available as:
0.5 mg:
White to off-white, oval, flat-faced, beveled-edge, scored tablet. Debossed with 899 / 1/2 on the scored side and stylized b on the other side, packaged in bottles of 100 (NDC 0555-0899-02).
1 mg:
Light purple, oval, flat-faced, beveled-edge, scored tablet. Debossed with 886 / 1 on the scored side and stylized b on the other side, packaged in bottles of 100 (NDC 0555-0886-02) and 500 (NDC 0555-0886-04).
2 mg:
Green, oval, flat-faced, beveled-edge, scored tablet. Debossed with 887 / 2 on the scored side and stylized b on the other side, packaged in bottles of 100 (NDC 0555-0887-02) and 500 (NDC 0555-0887-04).
Store at 20º to 25º C (68º to 77ºF) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Keep this and all medications out of the reach of children.
Manufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Rev. D 2/2024
Close -
PATIENT INFORMATIONEstradiol (es″ tra dye′ ol) Tablets - Read this PATIENT INFORMATION before you start taking estradiol tablets and read what you get each time you refill estradiol tablets. There may be new ...
Estradiol (es″ tra dye′ ol) Tablets
Read this PATIENT INFORMATION before you start taking estradiol tablets and read what you get each time you refill estradiol tablets. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT ESTRADIOL TABLETS (AN ESTROGEN HORMONE)?
- Estrogens increase the chances of getting cancer of the uterus.
Report any unusual vaginal bleeding right away while you are taking estrogens. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- Do not use estrogens with or without progestins to prevent heart disease, heart attacks, or strokes.
Using estrogens with or without progestins may increase your chances of getting heart attacks, strokes, breast cancer, and blood clots. Using estrogens with progestins may increase your risk of dementia. You and your healthcare provider should talk regularly about whether you still need treatment with estradiol tablets.
WHAT ARE ESTRADIOL TABLETS?
Estradiol tablets are a medicine that contains estrogen hormones.
WHAT IS ESTRADIOL USED FOR?
Estradiol is used to:
- reduce moderate to severe hot flashes
Estrogens are hormones made by a woman's ovaries. Between ages 45 and 55, the ovaries normally stop making estrogens. This leads to a drop in body estrogen levels which causes the “change of life” or menopause (the end of monthly menstrual periods). Sometimes, both ovaries are removed during an operation before natural menopause takes place. The sudden drop in estrogen levels causes “surgical menopause.”
When the estrogen levels begin dropping, some women develop very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest, or sudden strong feelings of heat and sweating (“hot flashes” or “hot flushes”). In some women, the symptoms are mild, and they will not need estrogens. In other women, symptoms can be more severe. You and your healthcare provider should talk regularly about whether you still need treatment with estradiol.
Weight-bearing exercise, like walking or running, and taking calcium with vitamin D supplements may also lower your chances for getting postmenopausal osteoporosis. It is important to talk about exercise and supplements with your healthcare provider before starting them.
- treat dryness, itching, and burning in or around the vagina, difficulty or burning on urination associated with menopause
You and your healthcare provider should talk regularly about whether you still need treatment with estradiol to control these problems. If you use estradiol only to treat your dryness, itching, and burning in and around your vagina, talk with your healthcare provider about whether a topical vaginal product would be better for you.
- treat certain conditions in which a young woman's ovaries do not produce enough estrogen naturally
- treat certain types of abnormal vaginal bleeding due to hormonal imbalance when your doctor has found no serious cause of the bleeding
- treat certain cancers in special situations, in men and women
- prevent thinning of bones
Osteoporosis from menopause is a thinning of the bones that makes them weaker and easier to break. If you use estradiol only to prevent osteoporosis from menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you. You and your healthcare provider should talk regularly about whether you should continue with estradiol.
WHO SHOULD NOT USE ESTRADIOL?
Do not start taking estradiol if you:
- have unusual vaginal bleeding which has not been evaluated by your doctor (see BOXED WARNINGS)
Unusual vaginal bleeding can be a warning sign of cancer of the uterus, especially if it happens after menopause. Your doctor must find out the cause of the bleeding so that he or she can recommend the proper treatment. Taking estrogens without visiting your doctor can cause you serious harm if your vaginal bleeding is caused by cancer of the uterus.
- currently have or have had certain cancers
Estrogens may increase the risk of certain types of cancer, including cancer of the breast or uterus. If you have or had cancer, talk with your healthcare provider about whether you should take estradiol.
(For certain patients with breast or prostate cancer, estrogens may help.)
- had a stroke or heart attack in the past year
- currently have or have had blood clots
- have or have had liver problems
- are allergic to estradiol tablets or any of its ingredients
See the end of this leaflet for a list of ingredients in estradiol tablets.
Estradiol tablets 2 mg contain tartrazine which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
- think you may be pregnant
Tell your healthcare provider:
- if you are breast feeding
The hormone in estradiol can pass into your milk
- about all of your medical problems
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), migraine, endometriosis, lupus, problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood.
- about all the medicines you take
This includes prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how estradiol tablets work. Estradiol tablets may also affect how your other medicines work.
- if you are going to have surgery or will be on bed rest
You may need to stop taking estrogens.
HOW SHOULD I TAKE ESTRADIOL TABLETS?
1. Start at the lowest dose and talk to your healthcare provider about how well that dose is working for you.
2. Estrogens should be used at the lowest dose possible for your treatment only as long as needed. You and your healthcare provider should talk regularly (for example, every 3 to 6 months) about the dose you are taking and whether you still need treatment with estradiol.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF ESTROGENS?
Less common but serious side effects include:
- Breast cancer
- Cancer of the uterus
- Stroke
- Heart attack
- Blood clots
- Dementia
- Gallbladder disease
- Ovarian cancer
These are some of the warning signs of the serious side effects:
- Breast lumps
- Unusual vaginal bleeding
- Dizziness and faintness
- Changes in speech
- Severe headaches
- Chest pain
- Shortness of breath
- Pains in your legs
- Changes in vision
- Vomiting
Call your healthcare provider right away if you get any of these warning signs, or any other unusual symptom that concerns you.
Common side effects include:
- Headache
- Breast pain
- Irregular vaginal bleeding or spotting
- Stomach/abdominal cramps, bloating
- Nausea and vomiting
- Hair loss
Other side effects include:
- High blood pressure
- Liver problems
- High blood sugar
- Fluid retention
- Enlargement of benign tumors (“fibroids”) of the uterus
- A spotty darkening of the skin, particularly on the face
- Vaginal yeast infection
These are not all the possible side effects of estradiol tablets. For more information, ask your healthcare provider or pharmacist.
WHAT CAN I DO TO LOWER MY CHANCES OF A SERIOUS SIDE EFFECT WITH ESTRADIOL?
If you use estrogens, you can reduce your risks by doing these things:
- Talk with your healthcare provider:
1. While you are using estrogens, it is important to visit your doctor at least once a year for a check-up.
2. If you have a uterus, talk to your healthcare provider about whether the addition of a progestin is right for you.
3. See your healthcare provider right away if you have vaginal bleeding while taking estradiol tablets.
4. Have a breast exam and mammogram (breast x-ray) every year unless your healthcare provider tells you something else. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram (breast x-ray), you may need to have more frequent breast examinations.
5. If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease. Ask your healthcare provider for ways to lower your chances for getting heart disease.
6. Talk with your healthcare provider regularly about whether you should continue taking estradiol tablets. You and your doctor should reevaluate whether or not you still need estrogens at least every six months.
- Be alert for signs of trouble
If any of these warning signals (or any other unusual symptoms) happen while you are using estrogens, call your doctor immediately:
Abnormal bleeding from the vagina (possible uterine cancer)
Pains in the calves or chest, sudden shortness of breath, or coughing blood (possible clot in the legs, or lungs)
Severe headache or vomiting, dizziness, faintness, changes in vision or speech, weakness or numbness of an arm or leg (possible clot in the brain or eye)
Breast lumps (possible breast cancer; ask your doctor or health professional to show you how to examine your breasts monthly)
Yellowing of the skin or eyes (possible liver problem)
Pain, swelling, or tenderness in the abdomen (possible gallbladder problem)
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
GENERAL INFORMATION ABOUT SAFE AND EFFECTIVE USE OF ESTRADIOL TABLETS
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets.
Do not take estradiol tablets for conditions for which they were not prescribed. Do not give estradiol tablets to other people, even if they have the same symptoms you have. They may harm them.
KEEP ESTRADIOL TABLETS OUT OF THE REACH OF CHILDREN
This leaflet provides a summary of the most important information about estradiol tablets. If you would like more information, talk with your healthcare provider or pharmacist. You can ask for information about estradiol tablets that is written for health professionals. For more information about estradiol, please contact Teva at 1-888-838-2872.
WHAT ARE THE INGREDIENTS IN ESTRADIOL TABLETS?
Inactive Ingredients: Colloidal silicon dioxide, corn starch, dibasic calcium phosphate, lactose monohydrate, magnesium stearate, and sodium starch glycolate. In addition, the 1 mg also contains FD&C blue no. 1 aluminum lake and D&C red no. 27 aluminum lake. The 2 mg also contains FD&C blue no. 1 aluminum lake and FD&C yellow no. 5 (tartrazine) aluminum lake.
Manufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Rev. B 11/2022
Close -
PRINCIPAL DISPLAY PANELNDC 0555-0899-02 - Estradiol Tablets, USP - 0.5 mg - PHARMACIST: Dispense the - accompanying Patient Information - Leaflet to each patient. Rx only - 100 Tablets
NDC 0555-0899-02
Estradiol Tablets, USP
0.5 mg
PHARMACIST: Dispense the
accompanying Patient Information
Leaflet to each patient.Rx only
100 Tablets
Close
-
PRINCIPAL DISPLAY PANELNDC 0555-0886-02 - Estradiol Tablets, USP - 1 mg - PHARMACIST: Dispense the - accompanying Patient Information - Leaflet to each patient. Rx only - 100 Tablets
NDC 0555-0886-02
Estradiol Tablets, USP
1 mg
PHARMACIST: Dispense the
accompanying Patient Information
Leaflet to each patient.Rx only
100 Tablets
Close
-
PRINCIPAL DISPLAY PANELNDC 0555-0887-02 - Estradiol Tablets, USP - 2 mg - PHARMACIST: Dispense the - accompanying Patient Information - Leaflet to each patient. Contains FD&C Yellow No. 5 - (tartrazine) as a color additive. Rx ...
NDC 0555-0887-02
Estradiol Tablets, USP
2 mg
PHARMACIST: Dispense the
accompanying Patient Information
Leaflet to each patient.Contains FD&C Yellow No. 5
(tartrazine) as a color additive.Rx only
100 Tablets
Close
-
INGREDIENTS AND APPEARANCEProduct Information
ESTRADIOL estradiol tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0555-0899 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white (white to off-white) Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code 899;1;2;b Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0555-0899-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040197 10/28/1997 ESTRADIOL estradiol tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0555-0886 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 1 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) Product Characteristics Color purple (light purple) Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code 886;1;b Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0555-0886-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/1997 2 NDC:0555-0886-04 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040197 10/28/1997 ESTRADIOL estradiol tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0555-0887 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 2 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color green Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code 887;2;b Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0555-0887-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/1997 2 NDC:0555-0887-04 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040197 10/28/1997
CloseLabeler - Teva Pharmaceuticals USA, Inc. (001627975)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ESTRADIOL tablet
Number of versions: 15
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Mar 8, 2024 | 15 (current) | download |
| Nov 21, 2023 | 14 | download |
| Nov 19, 2021 | 12 | download |
| Jun 17, 2021 | 11 | download |
| May 1, 2020 | 10 | download |
| Apr 29, 2020 | 9 | download |
| Nov 5, 2019 | 8 | download |
| Nov 5, 2018 | 7 | download |
| Jun 18, 2018 | 6 | download |
| Aug 4, 2016 | 5 | download |
| Oct 18, 2013 | 4 | download |
| Aug 22, 2012 | 3 | download |
| Jan 26, 2011 | 2 | download |
| Aug 21, 2006 | 1 | download |
| Apr 26, 2006 | (-1) |
Get Label RSS Feed for this Drug
ESTRADIOL tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=c4936878-1643-4e7f-9b0d-e4957935aef2
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ESTRADIOL tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0555-0886-02 |
| 2 | 0555-0886-04 |
| 3 | 0555-0887-02 |
| 4 | 0555-0887-04 |
| 5 | 0555-0899-02 |



