Label: MEGESTROL ACETATE tablet
- NDC Code(s): 0555-0606-02, 0555-0607-02
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
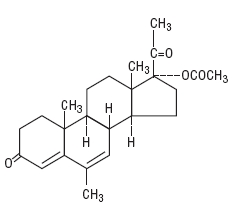
DESCRIPTIONMegestrol acetate tablets, USP are a synthetic, antineoplastic and progestational drug. Megestrol acetate is a white, crystalline solid chemically designated as ...
-
CLINICAL PHARMACOLOGYWhile the precise mechanism by which megestrol acetate produces its antineoplastic effects against endometrial carcinoma is unknown at the present time, inhibition of pituitary gonadotrophin ...
-
INDICATIONS AND USAGEMegestrol acetate tablets are indicated for the palliative treatment of advanced carcinoma of the breast or endometrium (i.e., recurrent, inoperable, or metastatic disease). It should not be used ...
-
CONTRAINDICATIONSHistory of hypersensitivity to megestrol acetate or any component of the formulation.
-
WARNINGSMegestrol acetate may cause fetal harm when administered to a pregnant woman. Fertility and reproduction studies with high doses of megestrol acetate have shown a reversible feminizing effect on ...
-
PRECAUTIONSGeneral - Close surveillance is indicated for any patient treated for recurrent or metastatic cancer. Use with caution in patients with a history of thromboembolic disease. Use in ...
-
ADVERSE REACTIONSWeight Gain - Weight gain is a frequent side effect of megestrol acetate. This gain has been associated with increased appetite and is not necessarily associated with fluid ...
-
OVERDOSAGENo serious unexpected side effects have resulted from studies involving megestrol acetate administered in dosages as high as 1600 mg/day. Oral administration of large, single doses of megestrol ...
-
DOSAGE AND ADMINISTRATIONBreast Cancer: 160 mg/day (40 mg qid). Endometrial Carcinoma: 40 to 320 mg/day in divided doses. At least 2 months of continuous treatment is considered an adequate period for determining the ...
-
HOW SUPPLIEDMegestrol acetate tablets, USP are available as: 20 mg: White, round, flat-faced, beveled-edge, scored tablet. Debossed with 555/606 on one side and stylized b on the other side. Available in ...
-
SPECIAL HANDLINGHealth Hazard Data - There is no threshold limit value established by OSHA, NIOSH, or ACGIH. Exposure or “overdose” at levels approaching recommended dosing levels could result in side effects ...
-
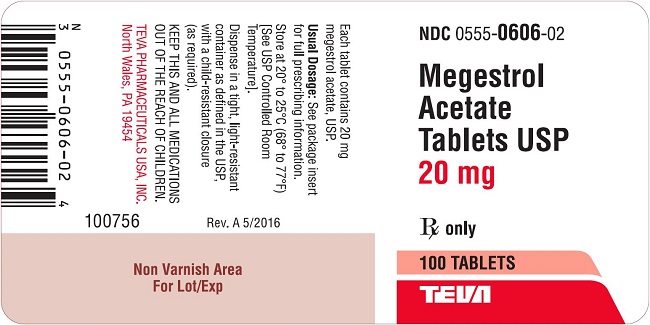
Package/Label Display PanelMegestrol Acetate Tablets USP 20 mg 100s Label Text - NDC 0555-0606-02 - Megestrol Acetate Tablets, USP - 20 mg - Rx only - 100 Tablets - teva
-
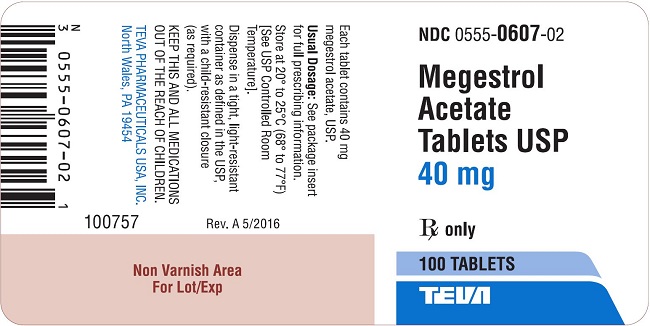
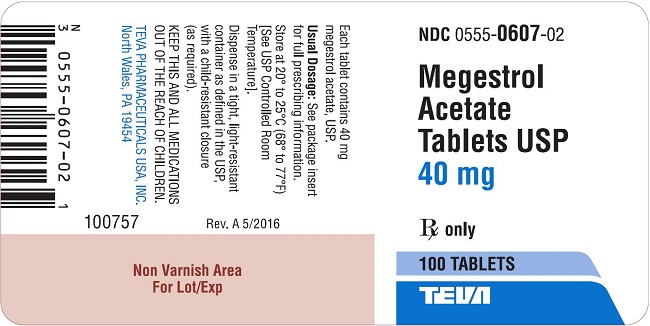
Package/Label Display PanelMegestrol Acetate Tablets USP 40 mg 100s Label Text - NDC 0555-0607-02 - Megestrol Acetate Tablets, USP - 40 mg - Rx only - 100 Tablets - teva
-
INGREDIENTS AND APPEARANCEProduct Information