Label: METHOTREXATE tablet
- NDC Code(s): 0555-0572-02, 0555-0572-35
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METHOTREXATE TABLETS safely and effectively. See full prescribing information for METHOTREXATE TABLETS. METHOTREXATE tablets ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY, HYPERSENSITIVITY REACTIONS AND SEVERE ADVERSE REACTIONS
-
Methotrexate can cause embryo-fetal toxicity, including fetal death. For non- neoplastic diseases, methotrexate is contraindicated in pregnancy. For neoplastic diseases, advise females and males of reproductive potential to use effective contraception [see Contraindications (4), Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
- Methotrexate is contraindicated in patients with a history of severe hypersensitivity reactions to methotrexate, including anaphylaxis [Contraindications (4), Warnings and Precautions (5.2)].
- Serious adverse reactions, including death, have been reported with methotrexate. Closely monitor for adverse reactions of the bone marrow, gastrointestinal tract, liver, lungs, skin, and kidneys. Withhold or discontinue methotrexate as appropriate [Warnings and Precautions (5.3, 5.4, 5.5, 5.6, 5.7, 5.8)].
-
Methotrexate can cause embryo-fetal toxicity, including fetal death. For non- neoplastic diseases, methotrexate is contraindicated in pregnancy. For neoplastic diseases, advise females and males of reproductive potential to use effective contraception [see Contraindications (4), Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
-
1 INDICATIONS AND USAGE1.1 Neoplastic Diseases - Methotrexate tablets are indicated for the: treatment of adults and pediatric patients with acute lymphoblastic leukemia (ALL) as part of a combination ...
-
2 DOSAGE AND ADMINISTRATION2.1 - Important Dosage and Safety Information - Verify pregnancy status in females of reproductive potential before starting methotrexate tablets [see Contraindications (4), Warnings and ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: Yellow, oval-shaped, film coated, scored, biconvex tablet. Debossed with b/572 on the scored side.
-
4 CONTRAINDICATIONSMethotrexate tablets are contraindicated in: Pregnant women receiving methotrexate tablets for treatment of non-neoplastic diseases [see Warnings and Precautions (5.1), and Use in Specific ...
-
5 WARNINGS AND PRECAUTIONS5.1 - Embryo-Fetal Toxicity - Based on published reports and its mechanism of action, methotrexate can cause fetal harm, including fetal death, when administered to a pregnant woman. Methotrexate ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.2)] Myelosuppression [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 - Effects of Other Drugs on Methotrexate - Drugs that Increase Methotrexate Exposure - Coadministration of methotrexate with the following products may increase methotrexate plasma ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Methotrexate is contraindicated in pregnant women with non-neoplastic diseases [see Contraindications (4)]. Based on published reports and its mechanism of action ...
-
10 OVERDOSAGEOverdosage, including fatal overdosage, has occurred with methotrexate [see Warnings and Precautions (5. 9)]. Manifestations - Manifestations of methotrexate overdosage include adverse reactions ...
-
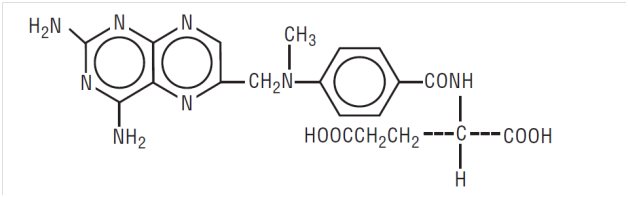
11 DESCRIPTIONMethotrexate, USP is dihydrofolate reductase inhibitor with the chemical name of L-glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]. The molecular formula is C20H22N8O5 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Methotrexate inhibits dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by this enzyme before they can be utilized as carriers of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Methotrexate has been evaluated in a number of animal studies for carcinogenic potential with inconclusive results. There is evidence ...
-
15 REFERENCES1. "OSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMethotrexate Tablets, USP are supplied as follows: 2.5 mg: Yellow, oval-shaped, film-coated, scored, biconvex tablet. Debossed with b/572 on the scored side. Available in bottles of 36 tablets ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Embryo-Fetal Toxicity - Advise females of reproductive potential of the potential risk to a fetus and to ...
-
SPL UNCLASSIFIED SECTIONDispense with Patient Package Insert available at: www.tevausa.com/PatientPI
-
PATIENT PACKAGE INSERTPATIENT INFORMATION ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANELNDC 0555-0572-35 - Methotrexate Tablets, USP - 2.5 mg - Caution: Cytotoxic Agent - PHARMACIST: Dispense the accompanying Patient Information Leaflet to each patient. Rx only - 36 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information