Label: LEUCOVORIN CALCIUM tablet
- NDC Code(s): 0555-0484-01, 0555-0484-02, 0555-0485-27
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
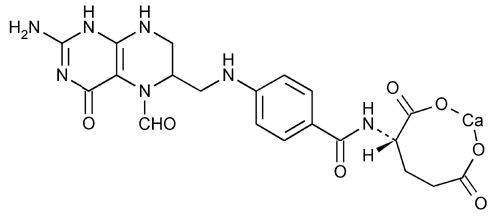
DESCRIPTIONLeucovorin calcium tablets, USP contain either 5 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1, 4, 5, 6, 7, 8-hexahydro-4-oxo-6-pteridinyl)methyl ...
-
CLINICAL PHARMACOLOGYLeucovorin is a racemic mixture of the diastereoisomers of the 5-formyl derivative of tetrahydrofolic acid. The biologically active compound of the mixture is the (-)-L-isomer, known as Citrovorum ...
-
INDICATIONS AND USAGELeucovorin calcium tablets are indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosages of folic acid antagonists.
-
CONTRAINDICATIONSLeucovorin is improper therapy for pernicious anemia and other megaloblastic anemias secondary to the lack of vitamin B12. A hematologic remission may occur while neurologic manifestations ...
-
WARNINGSIn the treatment of accidental overdosage of folic acid antagonists, leucovorin should be administered as promptly as possible. As the time interval between antifolate administration (e.g. ...
-
PRECAUTIONSGeneral - Parenteral administration is preferable to oral dosing if there is a possibility that the patient may vomit or not absorb the leucovorin. Leucovorin has no effect on other established ...
-
ADVERSE REACTIONSAllergic sensitization, including anaphylactoid reactions and urticaria, has been reported following the administration of both oral and parenteral leucovorin. To report SUSPECTED ADVERSE ...
-
OVERDOSAGEExcessive amounts of leucovorin may nullify the chemotherapeutic effect of folic acid antagonists.
-
DOSAGE AND ADMINISTRATIONLeucovorin calcium tablets are intended for oral administration. Because absorption is saturable, oral administration of doses greater than 25 mg is not recommended. Impaired Methotrexate ...
-
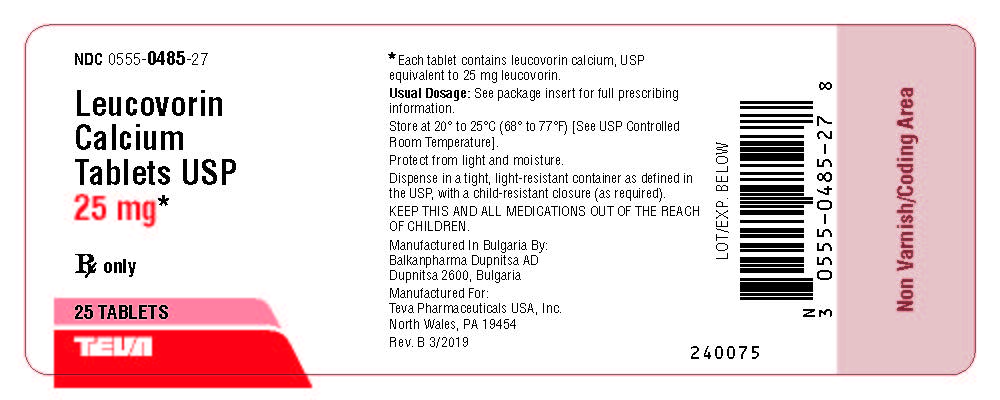
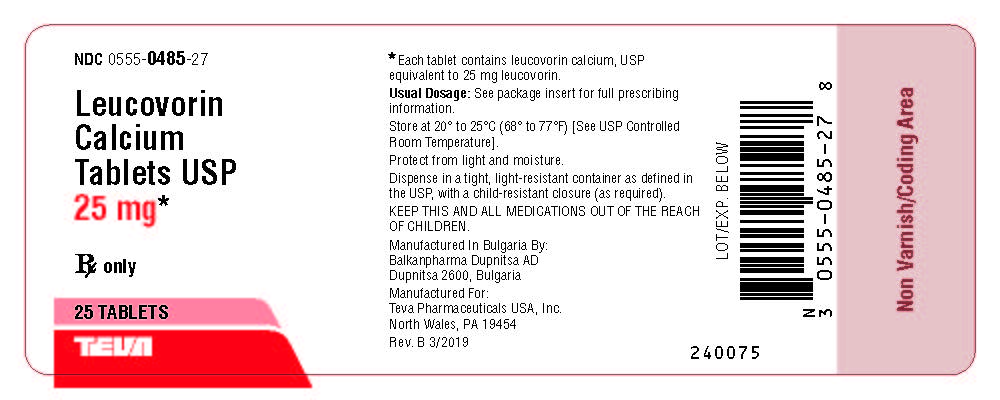
HOW SUPPLIEDLeucovorin calcium tablets USP, 5 mg are available as white, round, biconvex tablets, debossed with "stylized b" on one side and 484 on the other side, packaged in bottles of 30 (NDC 0555-0484-01 ...
-
REFERENCESGrem JL, Shoemaker DD, Petrelli NJ, Douglas HO. Severe and fatal toxic effects observed in treatment with high- and low-dose leucovorin plus 5-fluorouracil for colorectal carcinoma. Cancer Treat ...
-
Package/Label Display PanelNDC 0555-0484-01 - Leucovorin Calcium Tablets, USP - 5 mg* Rx only - 30 Tablets
-
Package/Label Display PanelNDC 0555-0485-27 - Leucovorin Calcium Tablets, USP - 25 mg* Rx only - 25 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information