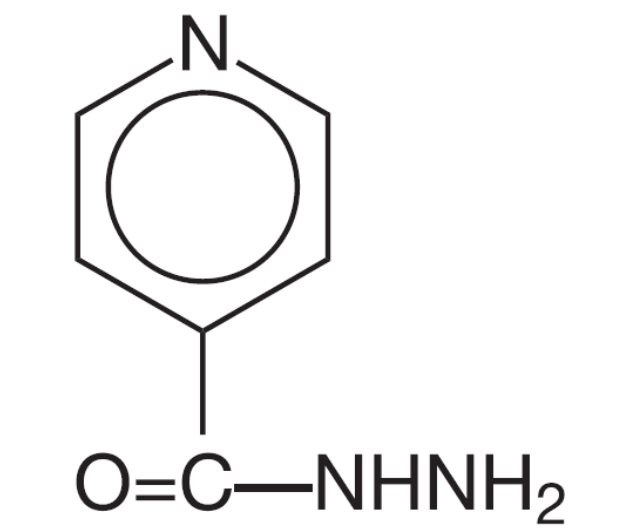
For preventive therapy of tuberculous infection and treatment of tuberculosis, it is recommended that physicians be familiar with the following publications: (1) the recommendations of the Advisory Council for the Elimination of Tuberculosis, published in the MMWR: vol 42; RR-4, 1993 and (2) Treatment of Tuberculosis and Tuberculosis Infection in Adults and Children, American Journal of Respiratory and Critical Care Medicine: vol 149; 1359-1374, 1994.
For Treatment of Tuberculosis
Isoniazid is used in conjunction with other effective anti-tuberculous agents. Drug susceptibility testing should be performed on the organisms initially isolated from all patients with newly diagnosed tuberculosis. If the bacilli becomes resistant, therapy must be changed to agents to which the bacilli are susceptible.
Usual Oral Dosage (depending on the regimen used):
Adults
5 mg/kg up to 300 mg daily in a single dose; or
15 mg/kg up to 900 mg/day, two or three times/week
Children
10 mg/kg to 15 mg/kg up to 300 mg daily in a single dose; or
20 mg/kg to 40 mg/kg up to 900 mg/day, two or three times/week
Patients with Pulmonary Tuberculosis Without HIV Infection
There are 3 regimen options for the initial treatment of tuberculosis in children and adults:
Option 1
Daily isoniazid, rifampin and pyrazinamide for 8 weeks followed by 16 weeks of isoniazid and rifampin daily or 2 to 3 times weekly. Ethambutol or streptomycin should be added to the initial regimen until sensitivity to isoniazid and rifampin is demonstrated. The addition of a fourth drug is optional if the relative prevalence of isoniazid-resistant Mycobacterium tuberculosis isolates in the community is less than or equal to four percent.
Option 2
Daily isoniazid, rifampin, pyrazinamide and streptomycin or ethambutol for 2 weeks followed by twice weekly administration of the same drugs for 6 weeks, subsequently twice weekly isoniazid and rifampin for 16 weeks.
Option 3
Three times weekly with isoniazid, rifampin, pyrazinamide and ethambutol or streptomycin for 6 months.
*All regimens given twice weekly or 3 times weekly should be administered by directly observed therapy
[see also Directly Observed Therapy (DOT)].
The above treatment guidelines apply only when the disease is caused by organisms that are susceptible to the standard antituberculous agents. Because of the impact of resistance to isoniazid and rifampin on the response to therapy, it is essential that physicians initiating therapy for tuberculosis be familiar with the prevalence of drug resistance in their communities. It is suggested that ethambutol not be used in children whose visual acuity cannot be monitored.
Patients with Pulmonary Tuberculosis and HIV Infection
The response of the immunologically impaired host to treatment may not be as satisfactory as that of a person with normal host responsiveness. For this reason, therapeutic decisions for the impaired host must be individualized. Since patients co-infected with HIV may have problems with malabsorption, screening of antimycobacterial drug levels, especially in patients with advanced HIV disease, may be necessary to prevent the emergence of MDRTB.
Patients with Extra Pulmonary Tuberculosis
The basic principles that underlie the treatment of pulmonary tuberculosis also apply to extra pulmonary forms of the disease. Although there have not been the same kinds of carefully conducted controlled trials of treatment of extra pulmonary tuberculosis as for pulmonary disease, increasing clinical experience indicates that a 6 to 9 month short-course regimen is effective. Because of the insufficient data, miliary tuberculosis, bone/joint tuberculosis and tuberculous meningitis in infants and children should receive 12 month therapy.
Bacteriologic evaluation of extra pulmonary tuberculosis may be limited by the relative inaccessibility of the sites of disease. Thus, response to treatment often must be judged on the basis of clinical and radiographic findings.
The use of adjunctive therapies such as surgery and corticosteroids is more commonly required in extra pulmonary tuberculosis than in pulmonary disease. Surgery may be necessary to obtain specimens for diagnosis and to treat such processes as constrictive pericarditis and spinal cord compression from Pott’s Disease. Corticosteroids have been shown to be of benefit in preventing cardiac constriction from tuberculous pericarditis and in decreasing the neurologic sequelae of all stages of tuberculosis meningitis, especially when administered early in the course of the disease.
Pregnant Women with Tuberculosis
The options listed above must be adjusted for the pregnant patient. Streptomycin interferes with in utero development of the ear and may cause congenital deafness. Routine use of pyrazinamide is also not recommended in pregnancy because of inadequate teratogenicity data. The initial treatment regimen should consist of isoniazid and rifampin. Ethambutol should be included unless primary isoniazid resistance is unlikely (isoniazid resistance rate documented to be less than 4%).
Treatment of Patients with Multi-Drug Resistant Tuberculosis (MDRTB)
Multiple-drug resistant tuberculosis (i.e., resistance to at least isoniazid and rifampin) presents difficult treatment problems. Treatment must be individualized and based on susceptibility studies. In such cases, consultation with an expert in tuberculosis is recommended.
Directly Observed Therapy (DOT)
A major cause of drug-resistant tuberculosis is patient noncompliance with treatment. The use of DOT can help assure patient compliance with drug therapy. DOT is the observation of the patient by a health care provider or other responsible person as the patient ingests anti-tuberculosis medications. DOT can be achieved with daily, twice weekly or thrice weekly regimens and is recommended for all patients.
For Preventative Therapy of Tuberculosis
Before isoniazid preventive therapy is initiated, bacteriologically positive or radiographically progressive tuberculosis must be excluded. Appropriate evaluations should be performed if extra pulmonary tuberculosis is suspected.
Adults over 30 kg: 300 mg per day in a single dose.
Infants and Children: 10 mg/kg (up to 300 mg daily) in a single dose. In situations where adherence with daily preventative therapy cannot be assured, 20 mg/kg to 30 mg/kg (not to exceed 900 mg) twice weekly under the direct observation of a health care worker at the time of administration8.
Continuous administration of isoniazid for a sufficient period is an essential part of the regimen because relapse rates are higher if chemotherapy is stopped prematurely. In the treatment of tuberculosis, resistant organisms may multiply and the emergence of resistant organisms during the treatment may necessitate a change in the regimen.
For following patient compliance: the Potts-Cozart test9, a simple colorimetric6 method of checking for isoniazid in the urine, is a useful tool for assuring patient compliance, which is essential for effective tuberculosis control. Additionally, isoniazid test strips are also available to check patient compliance.
Concomitant administration of pyridoxine (B6) is recommended in the malnourished and in those predisposed to neuropathy (e.g., alcoholics and diabetics).