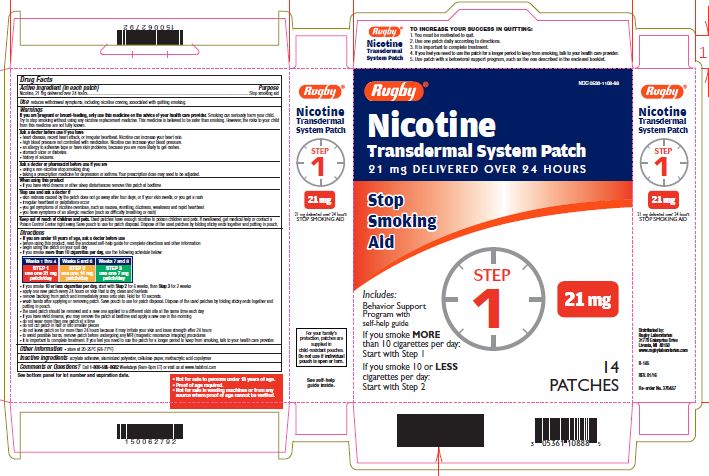
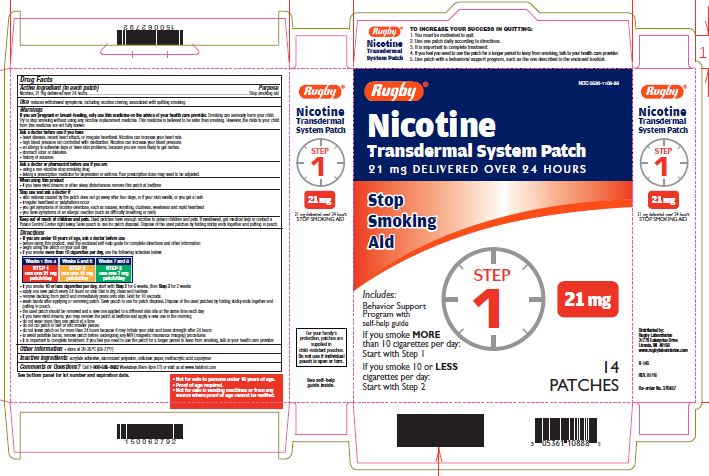
Label: NICOTINE TRANSDERMAL SYSTEM STEP 1- nicotine patch, extended release
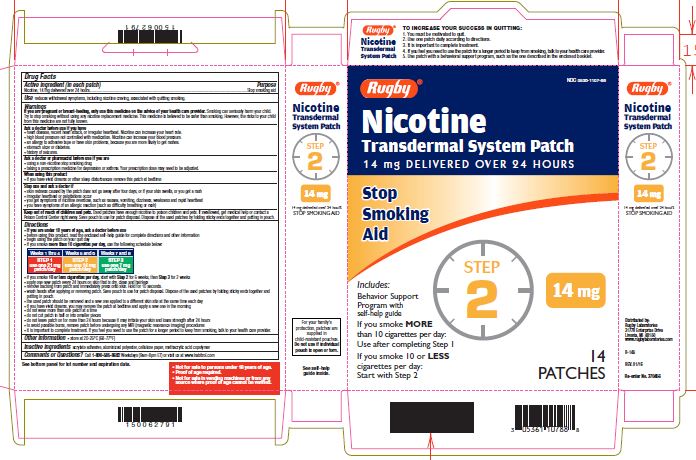
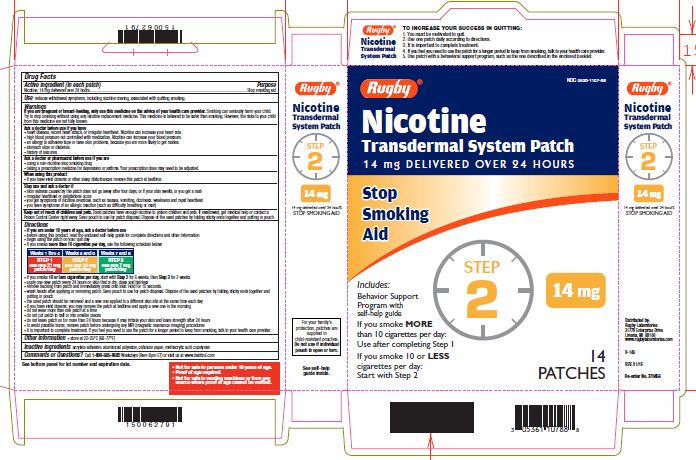
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, extended release
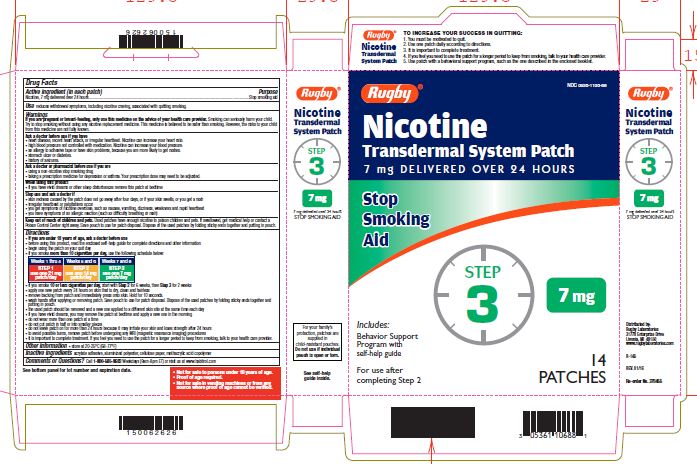
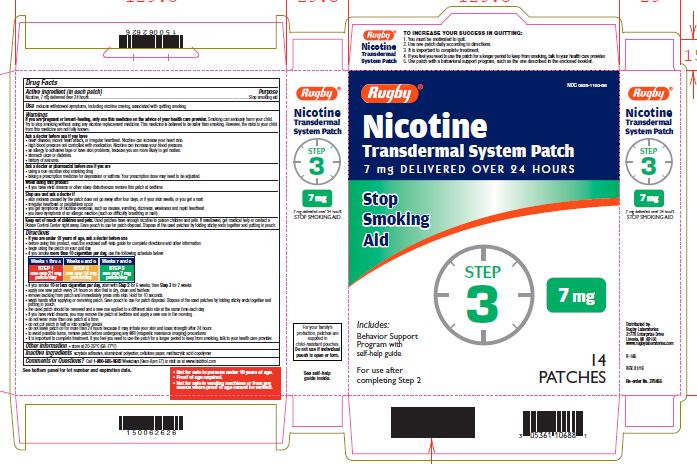
NICOTINE TRANSDERMAL SYSTEM STEP 3- nicotine patch, extended release
- NDC Code(s): 0536-1106-88, 0536-1107-88, 0536-1108-88
- Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient Step 1 (in each patch)
- Active ingredient Step 2 (in each patch)
- Active ingredient Step 3 (in each patch)
- Purpose
- Use
-
Warnings
If you are pregnant or breast-feeding, only use this medicine on the advice of your health care provider. Smoking can seriously harm your child. Try to stop smoking without using any nicotine replacement medicine. This medicine is believed to be safer than smoking. However, the risks to your child from this medicine are not fully known.
Ask a doctor before use if you have
- heart disease, recent heart attack, or irregular heartbeat. Nicotine can increase your heart rate.
- high blood pressure not controlled with medication. Nicotine can increase your blood pressure.
- an allergy to adhesive tape or have skin problems, because you are more likely to get rashes.
- stomach ulcer or diabetes.
- history of seizures.
Ask a doctor or pharmacist before use if you are
- •
- using a non-nicotine stop smoking drug
- •
- taking a prescription medicine for depression or asthma. Your prescription dose may need to be adjusted.
When using this product
- •
- if you have vivid dreams or other sleep disturbances remove this patch at bedtime
Stop use and ask a doctor if
- skin redness caused by the patch does not go away after four days, or if your skin swells, or you get a rash
- irregular heartbeat or palpitations occur
- you get symptoms of nicotine overdose, such as nausea, vomiting, dizziness, weakness and rapid heartbeat
- you have symptoms of an allergic reaction (such as difficulty breathing or rash)
Keep out of reach of children and pets.
Used patches have enough nicotine to poison children and pets. If swallowed, get medical help or contact a Poison Control Center right away. Save pouch to use for patch disposal. Dispose of the used patches by folding sticky ends together and putting in pouch.
-
Directions
- •
- if you are under 18 years of age, ask a doctor before use
- •
- before using this product, read the enclosed self-help guide for complete directions and other information
- •
- begin using the patch on your quit day
- •
- if you smoke more than 10 cigarettes per day, use the following schedule below:
STEP 1
STEP 2
STEP 3
Use one 21 mg patch/day
Use one 14 mg patch/day
Use one 7 mg patch/day
Weeks 1-4
Weeks 5-6
Weeks 7-8
- •
- if you smoke 10 or less cigarettes per day, start with Step 2 for 6 weeks, then Step 3 for 2 weeks
- •
- apply one new patch every 24 hours on skin that is dry, clean and hairless
- •
- remove backing from patch and immediately press onto skin. Hold for 10 seconds.
- •
- wash hands after applying or removing patch. Save pouch to use for patch disposal. Dispose of the used patches by folding sticky ends together and putting in pouch.
- •
- the used patch should be removed and a new one applied to a different skin site at the same time each day
- •
- if you have vivid dreams, you may remove the patch at bedtime and apply a new one in the morning
- •
- do not wear more than one patch at a time
- •
- do not cut patch in half or into smaller pieces
- •
- do not leave patch on for more than 24 hours because it may irritate your skin and loses strength after 24 hours
- •
- to avoid possible burns, remove patch before undergoing any MRI (magnetic resonance imaging) procedures
- •
- It is important to complete treatment. If you feel you need to use the patch for a longer period to keep from smoking, talk to your health care provider.
- Other information
- Inactive ingredients
-
Questions or comments?
call 1-800-585-8682
Weekdays (9am-8pm ET) or visit us at www.habitrol.com
TO INCREASE YOUR SUCCESS IN QUITTING:
1. You must be motivated to quit.
2. Use one patch daily according to directions.
3. It is important to complete treatment.
4. If you feel you need to use the patch for a longer period to keep from smoking, talk to your health care provider.
5. Use patch with a behavioural support program, such as the one described in the enclosed booklet.
For your family's protection, patches are supplied in child-resistant pouches. Do not use if individual pouch is open or torn.
- •
- Not for sale to persons under 18 years of age.
- •
- Proof of age required.
- •
- Not for sale in vending machines or from any source where proof of age cannot be verified.
- Principal Display Panel
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NICOTINE TRANSDERMAL SYSTEM STEP 1
nicotine patch, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1108 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICOTINE (UNII: 6M3C89ZY6R) (NICOTINE - UNII:6M3C89ZY6R) NICOTINE 21 mg in 24 h Inactive Ingredients Ingredient Name Strength Methacrylic Acid (UNII: 1CS02G8656) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1108-88 14 in 1 CARTON 04/01/2016 1 24 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020076 04/01/2016 NICOTINE TRANSDERMAL SYSTEM STEP 2
nicotine patch, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1107 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICOTINE (UNII: 6M3C89ZY6R) (NICOTINE - UNII:6M3C89ZY6R) NICOTINE 14 mg in 24 h Inactive Ingredients Ingredient Name Strength Methacrylic Acid (UNII: 1CS02G8656) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1107-88 14 in 1 CARTON 04/01/2016 1 24 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020076 04/01/2016 NICOTINE TRANSDERMAL SYSTEM STEP 3
nicotine patch, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1106 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICOTINE (UNII: 6M3C89ZY6R) (NICOTINE - UNII:6M3C89ZY6R) NICOTINE 7 mg in 24 h Inactive Ingredients Ingredient Name Strength Methacrylic Acid (UNII: 1CS02G8656) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1106-88 14 in 1 CARTON 04/01/2016 1 24 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020076 04/01/2016 Labeler - Rugby Laboratories (079246066)