Label: METOPROLOL SUCCINATE tablet, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 0527-2600-37, 0527-2600-43, 0527-2601-37, 0527-2601-43, view more - Packager: Lannett Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated October 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Metoprolol Succinate safely and effectively. See full prescribing information for Metoprolol Succinate. Metoprolol Succinate Tablet ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ISCHEMIC HEART DISEASE:
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate extended-release tablets, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 - 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol succinate extended-release tablet administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Warn patients against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol succinate extended-release tablet therapy abruptly even in patients treated only for hypertension (5.1).
Close -
1 INDICATIONS AND USAGE1.1 Hypertension - Metoprolol succinate is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular ...
-
2 DOSAGE AND ADMINISTRATIONMetoprolol succinate extended-release tablets are intended for once daily administration. For treatment of hypertension and angina, when switching from immediate-release metoprolol to metoprolol ...
-
3 DOSAGE FORMS AND STRENGTHS25 mg tablets White, oval, biconvex, film-coated scored tablet engraved with “A/β”. 50 mg tablets: White, round, biconvex, film-coated scored tablet engraved with “A/mo”. 100 mg tablets: White ...
-
4 CONTRAINDICATIONSMetoprolol succinate extended-release tablet is contraindicated in severe bradycardia, second or third degree heart block, cardiogenic shock, decompensated cardiac failure, sick sinus syndrome ...
-
5 WARNINGS AND PRECAUTIONS5.1 Ischemic Heart Disease - Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in labeling: • Worsening angina or myocardial infarction. [see Warnings and Precautions (5)] • Worsening heart failure. [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Catecholamine Depleting Drugs - Catecholamine depleting drugs (e.g. reserpine, monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - Metoprolol tartrate has been shown to increase post-implantation loss and decrease neonatal survival in rats at doses up to 22 times, on a mg/m2 basis, the ...
-
10 OVERDOSAGESigns and Symptoms - Overdosage of metoprolol succinate extended-release tablets may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include ...
-
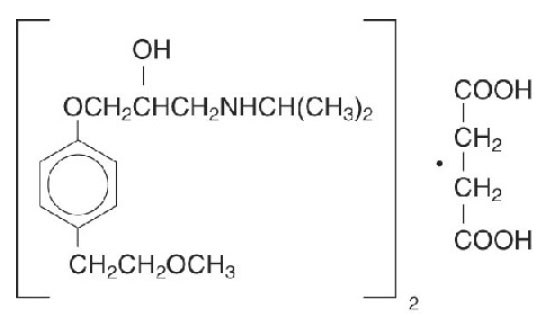
11 DESCRIPTIONMetoprolol succinate, is a beta1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. Metoprolol succinate extended-release ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Hypertension: The mechanism of the antihypertensive effects of beta-blocking agents has not been elucidated. However, several possible mechanisms have been proposed ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in ...
-
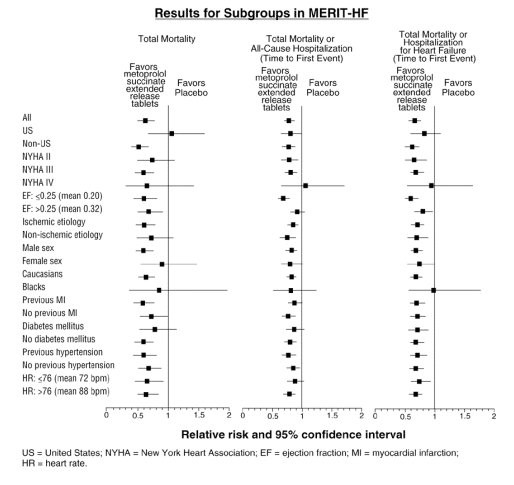
14 CLINICAL STUDIESIn five controlled studies in normal healthy subjects, the same daily doses of metoprolol succinate extended-release tablets and immediate-release metoprolol were compared in terms of the extent ...
-
15 REFERENCES1. Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Tablets containing metoprolol succinate equivalent to the indicated weight of metoprolol tartrate, USP, are white, biconvex, film-coated, and scored. Tablet - Shape - Engraving - Bottle of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to take metoprolol succinate extended–release tablets regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 25 mg NDC 0527-2600-37 - 100 Tablets - Metoprolol - Succinate - Extended-release Tablets - 25 mg* Rx only - Mfd. by: AstraZeneca AB - SE-151 85 Södertälje, Sweden - Mfd. for: Lannett Company, Inc. Philadelphia, PA ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 50 mg NDC 0527-2601-37 - 100 Tablets - Metoprolol - Succinate - Extended-release Tablets - 50 mg* Rx only - Mfd. by: AstraZeneca AB - SE-151 85 Södertälje, Sweden - Mfd. for: Lannett Company, Inc. Philadelphia, PA ...
-
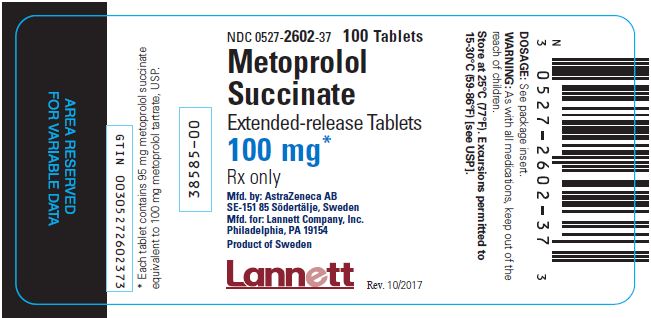
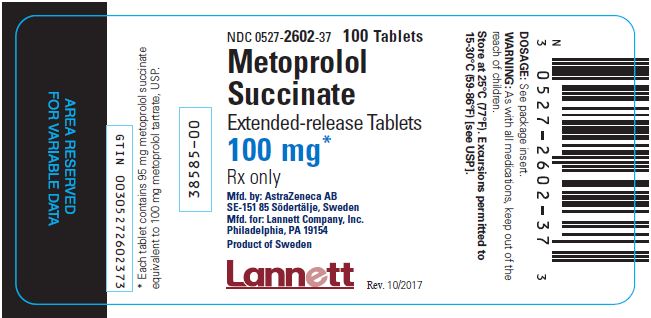
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 100 mg NDC 0527-2602-37 - 100 Tablets - Metoprolol - Succinate - Extended-release Tablets - 100 mg* Rx only - Mfd. by: AstraZeneca AB - SE-151 85 Södertälje, Sweden - Mfd. for: Lannett Company ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 200 mg NDC 0527-2603-37 - 100 Tablets - Metoprolol - Succinate - Extended-release Tablets - 200 mg* Rx only - Mfd. by: AstraZeneca AB - SE-151 85 Södertälje, Sweden - Mfd. for: Lannett Company, Inc. Philadelphia, PA ...
-
INGREDIENTS AND APPEARANCEProduct Information