Label: DANAZOL capsule
- NDC Code(s): 0527-1368-01, 0527-1369-01, 0527-1369-06, 0527-1392-01
- Packager: Lannett Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 30, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
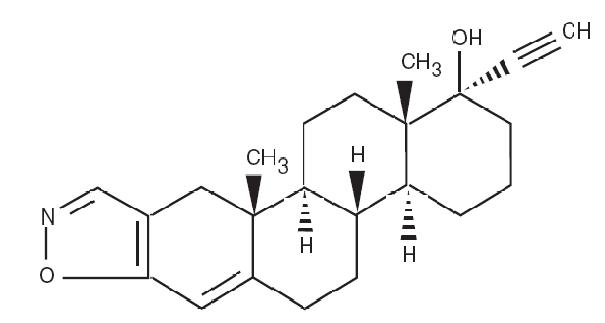
DESCRIPTIONDanazol is a synthetic steroid derived from ethisterone. It is a white to pale yellow crystalline powder, practically insoluble or insoluble in water, and sparingly soluble in alcohol. Chemically ...
-
CLINICAL PHARMACOLOGYDanazol suppresses the pituitary-ovarian axis. This suppression is probably a combination of depressed hypothalamic-pituitary response to lowered estrogen production, the alteration of sex steroid ...
-
INDICATIONS AND USAGEEndometriosis. Danazol capsules are indicated for the treatment of endometriosis amenable to hormonal management. Hereditary Angioedema. Danazol capsules are indicated for the prevention of ...
-
CONTRAINDICATIONSDanazol capsules should not be administered to patients with: Undiagnosed abnormal genital bleeding. Markedly impaired hepatic, renal, or cardiac function. Pregnancy (see WARNINGS). Breast ...
-
WARNINGSUse of danazol in pregnancy is contraindicated. A sensitive test (e.g., beta subunit test if available) capable of determining early pregnancy is recommended immediately prior to start of ...
-
PRECAUTIONSBecause danazol capsules may cause some degree of fluid retention, conditions that might be influenced by this factor, such as epilepsy, migraine, or cardiac or renal dysfunction, polycythemia and ...
-
ADVERSE REACTIONSThe following events have been reported in association with the use of danazol capsules: Androgen like effects include weight gain, acne and seborrhea. Mild hirsutism, edema, hair loss, voice ...
-
DOSAGE AND ADMINISTRATIONEndometriosis. In moderate to severe disease, or in patients infertile due to endometriosis, a starting dose of 800 mg given in two divided doses is recommended. Amenorrhea and rapid response to ...
-
HOW SUPPLIEDDanazol Capsules USP, 50 mg are available as maize opaque/white opaque capsules imprinted with logo "LANNETT" on the cap and "1392" on the body and are supplied in: Bottles of 100 (NDC ...
-
PRINCIPAL DISPLAY PANEL - 50 mgNDC 0527-1392-01 - Danazol Capsules, USP - 50 mg - Rx Only - 100 Capsules - Lannett
-
PRINCIPAL DISPLAY PANEL - 100 mgNDC-0527-1368-01 - Danazol Capsules, USP - 100 mg - Rx Only - 100 Capsules - Lannett
-
PRINCIPAL DISPLAY PANEL - 200 mgNDC-0527-1369-01 - Danazol Capsules, USP - 200 mg - Rx Only - 100 Capsules - Lannett
-
INGREDIENTS AND APPEARANCEProduct Information