Label: IPRATROPIUM BROMIDE solution
- NDC Code(s): 0487-9801-01, 0487-9801-02, 0487-9801-25, 0487-9801-30, view more
- Packager: Nephron Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
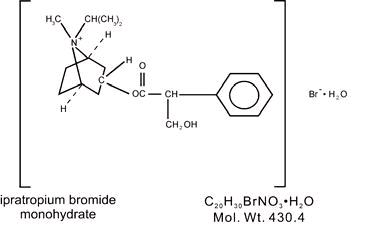
The active ingredient in ipratropium bromide inhalation solution is ipratropium bromide monohydrate. It is an anticholinergic bronchodilator chemically described as 8-Azoniabicyclo ...
-
CLINICAL PHARMACOLOGY
Ipratropium bromide is an anticholinergic (parasympatholytic) agent that, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action of acetylcholine, the ...
-
INDICATIONS AND USAGE
Ipratropium bromide inhalation solution administered either alone or with other bronchodilators, especially beta adrenergics, is indicated as a bronchodilator for maintenance treatment of ...
-
CONTRAINDICATIONS
Ipratropium bromide is contraindicated in known or suspected cases of hypersensitivity to ipratropium bromide, or to atropine and its derivatives.
-
WARNINGS
The use of ipratropium bromide inhalation solution as a single agent for the relief of bronchospasm in acute COPD exacerbation has not been adequately studied. Drugs with faster onset of action ...
-
PRECAUTIONS
General - Ipratropium bromide should be used with caution in patients with narrow angle glaucoma, prostatic hypertrophy or bladder neck obstruction. Information for Patients - Patients ...
-
ADVERSE REACTIONS
Adverse reaction information concerning ipratropium bromide inhalation solution is derived from 12-week active-controlled clinical trials. Additional information is derived from foreign ...
-
OVERDOSAGE
Acute systemic overdosage by inhalation is unlikely since ipratropium bromide is not well absorbed after inhalation at up to four-fold the recommended dose, or after oral administration at up to ...
-
DOSAGE AND ADMINISTRATION
The usual dosage of ipratropium bromide inhalation solution is 500 mcg (1 Unit-Dose Vial) administered three to four times a day by oral nebulization, with doses 6 to 8 hours apart. Ipratropium ...
-
HOW SUPPLIED
Ipratropium bromide inhalation solution USP is a clear, colorless solution containing 2.5 mL, packaged in cartons as listed below: NDC 0487-9801-25, 25 vials per carton / 5 vials per foil ...
-
Patient Package Insert
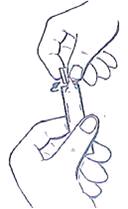
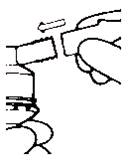
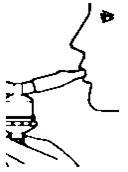
Patient's Instructions for Use - Ipratropium Bromide Inhalation Solution, USP 0.02% Read complete instructions carefully before using. Twist open the top of one unit dose vial and ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel – Pouch Label (5 Vial Card) NDC 0487-9801-25, NDC 0487-9801-30 and NDC 0487-9801-60 - Principal Display Panel – Carton (25 Count) NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information