Label: GRISEOFULVIN suspension
- NDC Code(s): 0472-0013-04
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
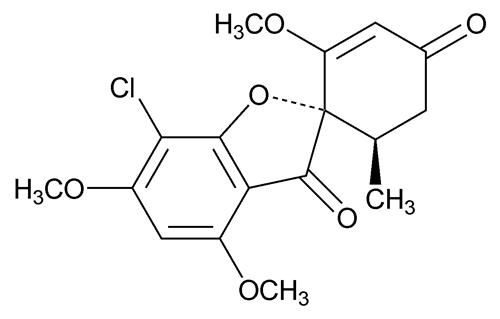
Griseofulvin microsize contains griseofulvin microsize for oral administration. The active ingredient, griseofulvin, USP is a fungistatic antibiotic, derived from a species of Penicillium. The ...
-
CLINICAL PHARMACOLOGYGriseofulvin absorption from the gastrointestinal tract varies considerably among individuals mainly because of insolubility of the drug in aqueous media of the upper GI tract. Drug absorption has ...
-
INDICATIONS AND USAGE
Griseofulvin oral suspension, USP is indicated for the treatment of dermatophyte infections of the skin not adequately treated by topical therapy, hair and nails, namely: Tinea corporis - Tinea ...
-
CONTRAINDICATIONS
Griseofulvin is contraindicated in patients with porphyria or hepatocellular failure, and in individuals with a history of hypersensitivity to griseofulvin. Griseofulvin may cause fetal harm when ...
-
WARNINGS
Prophylactic Usage: Safety and efficacy of griseofulvin for prophylaxis of fungal infections have not been established. Serious Skin Reactions: Severe skin reactions (e.g. Stevens-Johnson ...
-
PRECAUTIONS
General: Patients on prolonged therapy with any potent medication should be under close observation. Periodic monitoring of organ system function, including renal, hepatic and hematopoietic ...
-
ADVERSE REACTIONS
There have been postmarketing reports of severe skin and hepatic adverse events associated with griseofulvin use (see WARNINGS section). When adverse reactions occur, they are most commonly of ...
-
OVERDOSAGE
There is limited experience on overdose with griseofulvin. In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures as required.
-
DOSAGE AND ADMINISTRATION
Accurate diagnosis of the infecting organism is essential. Identification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium ...
-
HOW SUPPLIED
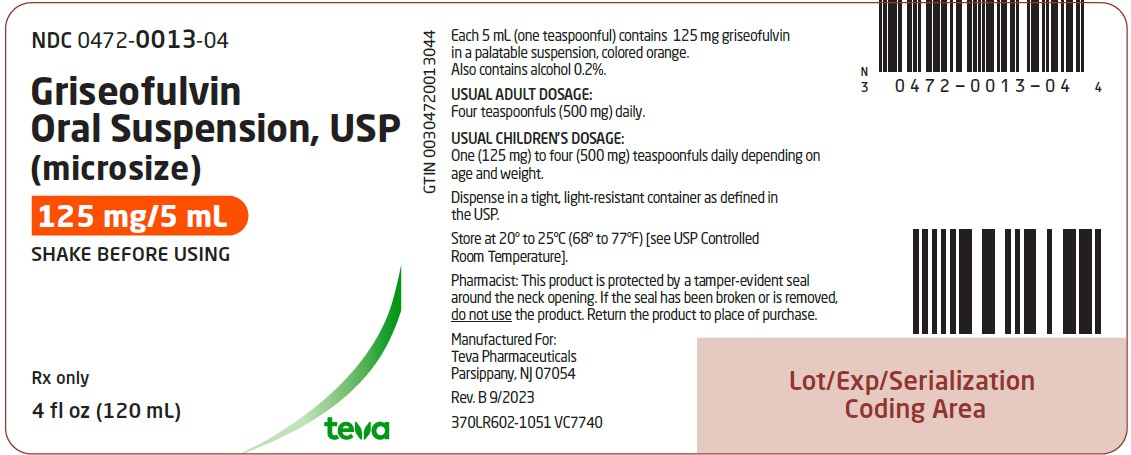
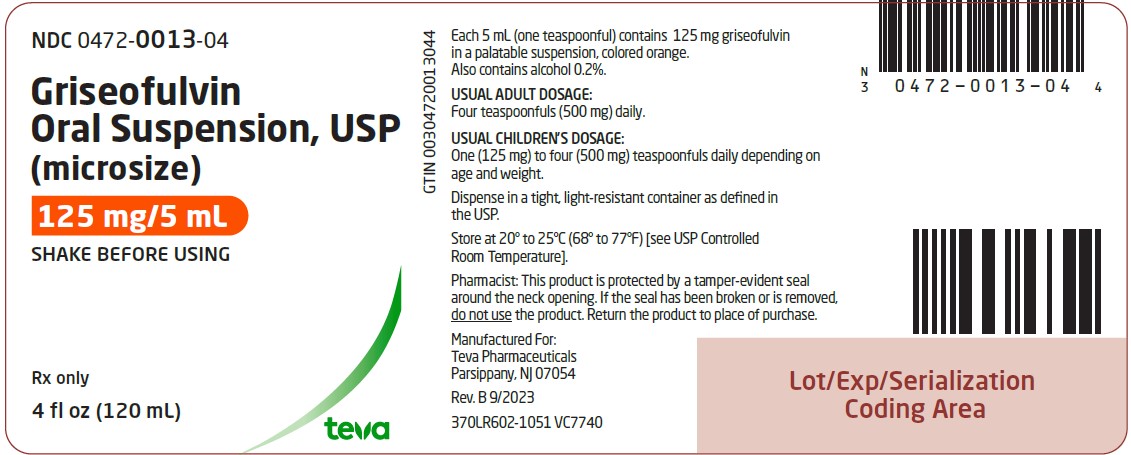
Griseofulvin oral suspension, USP (microsize), 125 mg per 5 mL is an orange-vanilla flavored suspension available in bottles of 4 fl oz (120 mL) NDC 0472-0013-04. Dispense griseofulvin oral ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0472-0013-04 - Griseofulvin Oral Suspension, USP - (microsize) 125 mg/5 mL - SHAKE BEFORE USING - Rx only - 4 fl oz (120 mL)
-
INGREDIENTS AND APPEARANCEProduct Information