Label: ACETYLCYSTEINE solution
- NDC Code(s): 0409-3307-03, 0409-3307-11, 0409-3308-03, 0409-3308-11
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONWARNING: NOT FOR INJECTION - Rx only
-
DESCRIPTION
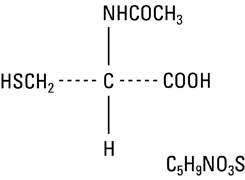
Acetylcysteine solution is for inhalation (mucolytic agent) or oral administration (acetaminophen antidote), and available as sterile, unpreserved solutions (not for injection). Acetylcysteine is ...
-
CLINICAL PHARMACOLOGY
The viscosity of pulmonary mucous secretions depends on the concentrations of mucoprotein and to a lesser extent deoxyribonucleic acid (DNA). The latter increases with increasing purulence owing ...
-
INDICATIONS AND USAGE
Acetylcysteine is indicated as adjuvant therapy for patients with abnormal, viscid, or inspissated mucous secretions in such conditions as: Chronic bronchopulmonary disease - (chronic emphysema ...
-
CONTRAINDICATIONS
Acetylcysteine is contraindicated in those patients who are sensitive to it.
-
WARNINGS
After proper administration of acetylcysteine, an increased volume of liquified bronchial secretions may occur. When cough is inadequate, the open airway must be maintained by mechanical suction ...
-
PRECAUTIONS
General - With the administration of acetylcysteine, the patient may initially notice a slight disagreeable odor that is soon noticeable. With a face mask there may be a stickiness on the face ...
-
ADVERSE REACTIONS
Adverse effects have included stomatitis, nausea, vomiting, fever, rhinorrhea, drowsiness, clamminess, chest tightness, and bronchoconstriction. Clinically overt acetylcysteine induced ...
-
DOSAGE AND ADMINISTRATION
General - Acetylcysteine Solution 10% and 20% is available in glass vials containing 30 mL. The 20% solution may be diluted to a lesser concentration with either Sodium Chloride Inhalation ...
-
CLINICAL PHARMACOLOGY
(Antidotal) Acetaminophen is rapidly absorbed from the upper gastrointestinal tract with peak plasma levels occurring between 30 and 60 minutes after therapeutic doses and usually within 4 hours ...
-
INDICATIONS AND USAGE
Acetylcysteine, administered orally, is indicated as an antidote to prevent or lessen hepatic injury which may occur following the ingestion of a potentially hepatotoxic quantity of ...
-
CONTRAINDICATIONS
There are no contraindications to oral administration of acetylcysteine in the treatment of acetaminophen overdose.
-
WARNINGS
Generalized urticaria has been observed rarely in patients receiving oral acetylcysteine for acetaminophen overdose. If this occurs or other allergic symptoms appear, treatment with acetylcysteine ...
-
PRECAUTIONS
Occasionally severe and persistent vomiting occurs as a symptom of acute acetaminophen overdose. Treatment with oral acetylcysteine may aggravate the vomiting. Patients at risk of gastric ...
-
ADVERSE REACTIONS
Oral administration of acetylcysteine, especially in the large doses needed to treat acetaminophen overdose, may result in nausea, vomiting and other gastrointestinal symptoms. Rash with or ...
-
DOSAGE AND ADMINISTRATION
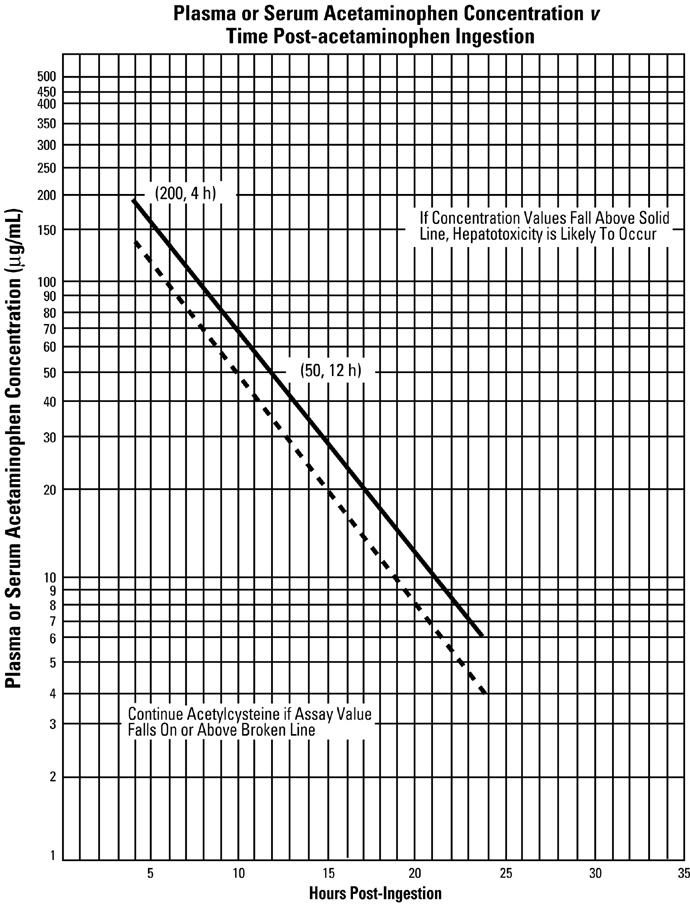
General - Regardless of the quantity of acetaminophen reported to have been ingested, administer acetylcysteine immediately if 24 hours or less have elapsed from the reported time of ingestion of ...
-
HOW SUPPLIED
Acetylcysteine Solution, USP is supplied in teartop vials as follows: Unit of SaleConcentration - NDC 0409-3307-03 - Carton containing 3 teartop vials - 10% 3 g/30 mL (100 mg/mL) NDC ...
-
REFERENCES
• Bonanomi L, Gazzaniga A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur J Respir Dis 1981; 61(suppl 111):45−51. • Am Rev Respir Dis 1960; 82:627−639. Distributed ...
-
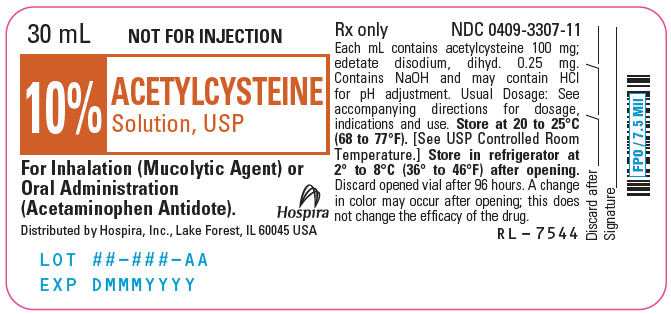
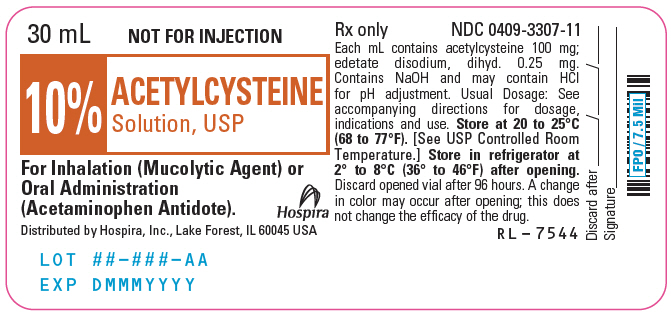
PRINCIPAL DISPLAY PANEL - 30 mL Vial Label - 10%30 mL - NOT FOR INJECTION - 10% ACETYLCYSTEINE - Solution, USP - For Inhalation (Mucolytic Agent) or - Oral Administration - (Acetaminophen Antidote). Distributed by Hospira, Inc., Lake Forest, IL 60045 ...
-
PRINCIPAL DISPLAY PANEL - 30 mL Vial Carton - 10%30 mL - Rx only - NDC 0409-3307-03 - Contains 3 of NDC 0409-3307-11 - 10% ACETYLCYSTEINE - Solution, USP - For Inhalation (Mucolytic Agent) or Oral Administration - (Acetaminophen Antidote). NOT FOR ...
-
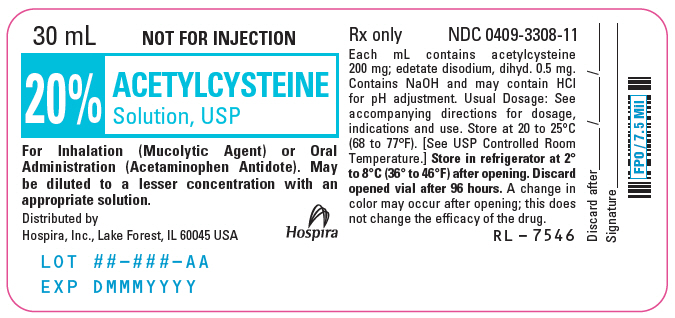
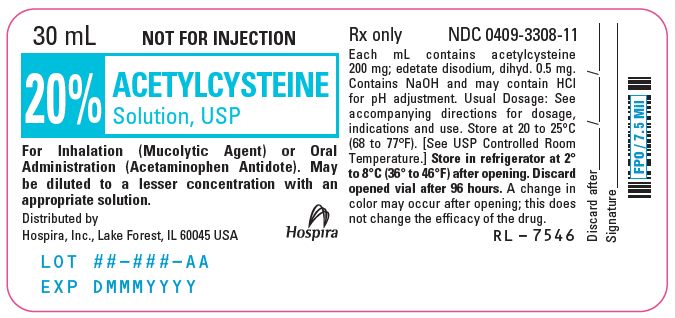
PRINCIPAL DISPLAY PANEL - 30 mL Vial Label - 20%30 mL - NOT FOR INJECTION - 20% ACETYLCYSTEINE - Solution, USP - For Inhalation (Mucolytic Agent) or Oral - Administration (Acetaminophen Antidote). May - be diluted to a lesser concentration with an ...
-
PRINCIPAL DISPLAY PANEL - 30 mL Vial Carton - 20%30 mL - Rx only - NDC 0409-3308-03 - Contains 3 of NDC 0409-3308-11 - 20% Acetylcysteine - Solution, USP - For Inhalation (Mucolytic Agent) or Oral Administration (Acetaminophen Antidote). NOT FOR ...
-
INGREDIENTS AND APPEARANCEProduct Information