Label: CYCLOSPORINE emulsion
- NDC Code(s): 0378-8760-58, 0378-8760-91, 0378-8760-98
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CYCLOSPORINE OPHTHALMIC EMULSION 0.05% safely and effectively. See full prescribing information for CYCLOSPORINE OPHTHALMIC ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Cyclosporine ophthalmic emulsion is indicated to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with ...
-
2 DOSAGE AND ADMINISTRATION Invert the unit dose vial a few times to obtain a uniform, white, opaque emulsion before using. Instill one drop of cyclosporine ophthalmic emulsion twice a day in each eye approximately 12 hours ...
-
3 DOSAGE FORMS AND STRENGTHS Ophthalmic emulsion containing cyclosporine 0.5 mg/mL
-
4 CONTRAINDICATIONS Cyclosporine ophthalmic emulsion is contraindicated in patients with known or suspected hypersensitivity to any of the ingredients in the formulation.
-
5 WARNINGS AND PRECAUTIONS 5.1 Potential for Eye Injury and Contamination Be careful not to touch the vial tip to your eye or other surfaces to avoid potential for eye injury and contamination. 5.2 Use with ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are described elsewhere in the labeling: • Potential for Eye Injury and Contamination [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Clinical administration of cyclosporine ophthalmic emulsion 0.05% is not detected systemically following topical ocular administration [see Clinical ...
-
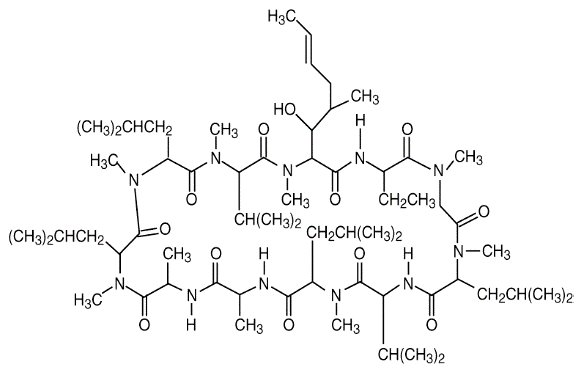
11 DESCRIPTION Cyclosporine ophthalmic emulsion 0.05% contains a topical calcineurin inhibitor immunosuppressant with anti-inflammatory effects. Cyclosporine’s chemical name is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Cyclosporine is an immunosuppressive agent when administered systemically. In patients whose tear production is presumed to be suppressed due to ocular inflammation ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Systemic carcinogenicity studies were conducted in male and female mice and rats. In the 78-week oral (diet) mouse ...
-
14 CLINICAL STUDIES Four multicenter, randomized, adequate and well-controlled clinical studies were performed in approximately 1,200 patients with moderate to severe keratoconjunctivitis sicca. Cyclosporine ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Cyclosporine ophthalmic emulsion, 0.05% is packaged in sterile, preservative-free single-use vials. Each vial contains 0.4 mL fill in a 0.5 mL natural colored low density polyethylene vial; five ...
-
17 PATIENT COUNSELING INFORMATION Handling the Container - Advise patients to not allow the tip of the vial to touch the eye or any surface, as this may contaminate the emulsion. Advise patients to not touch the vial tip to their ...
-
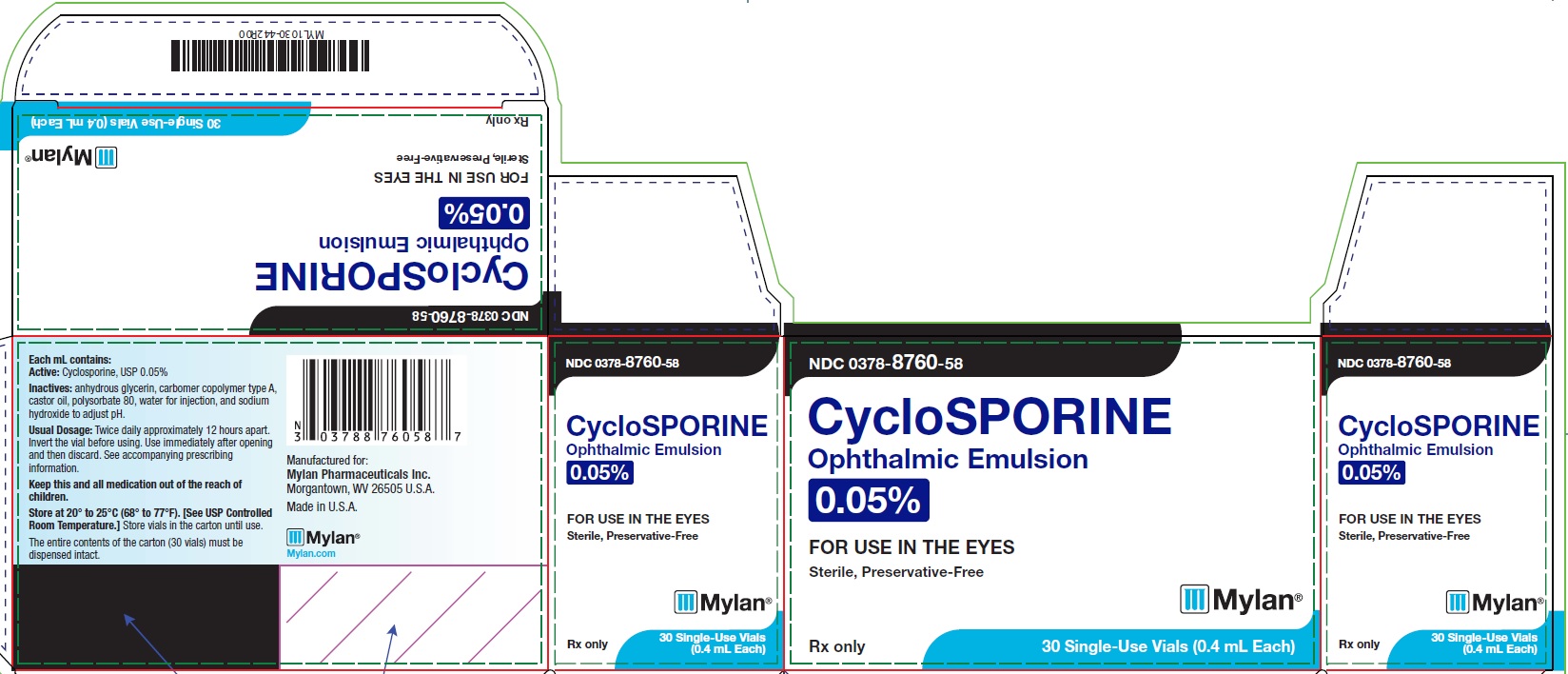
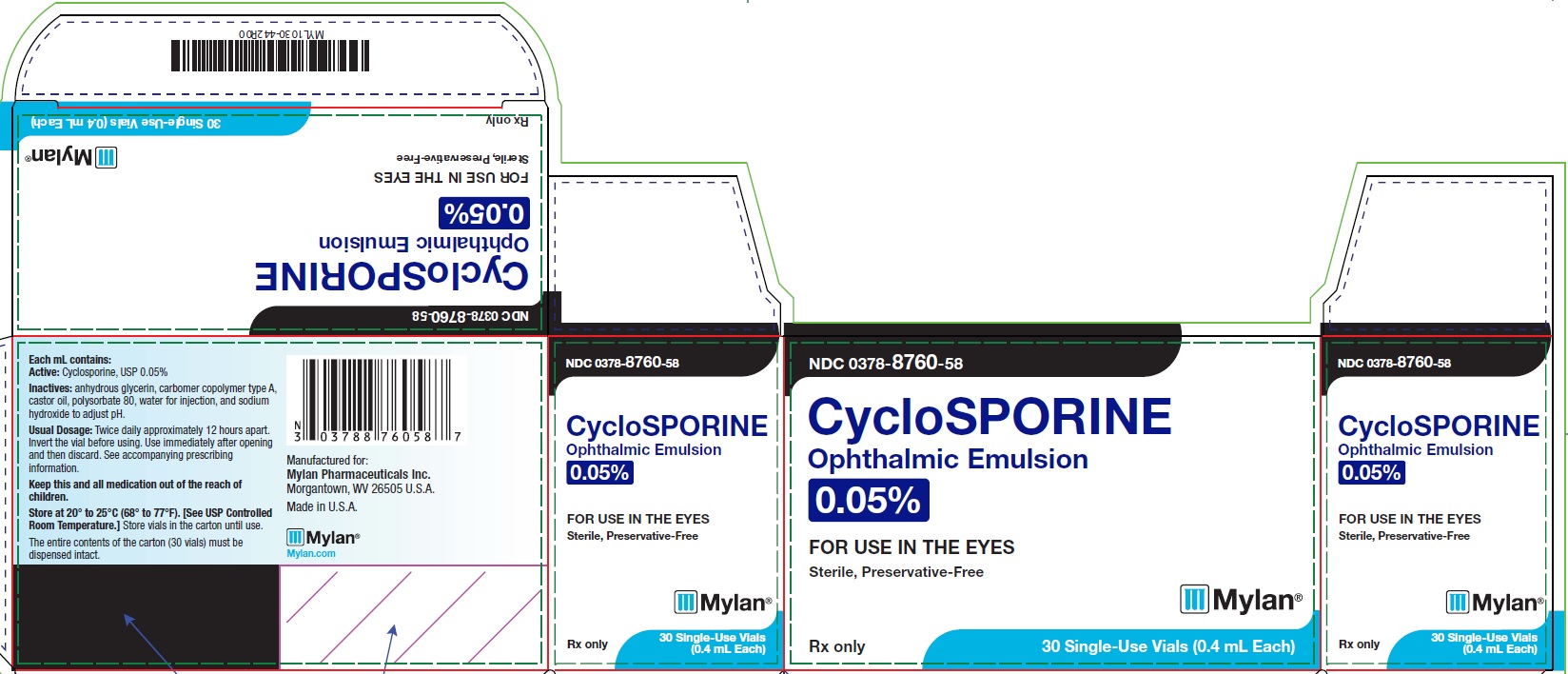
PRINCIPAL DISPLAY PANEL – 0.05% NDC 0378-8760-58 - CycloSPORINE - Ophthalmic Emulsion - 0.05% FOR USE IN THE EYES - Sterile, Preservative-Free - Rx only 30 Single-Use Vials (0.4 mL Each) Each mL contains: Active ...
-
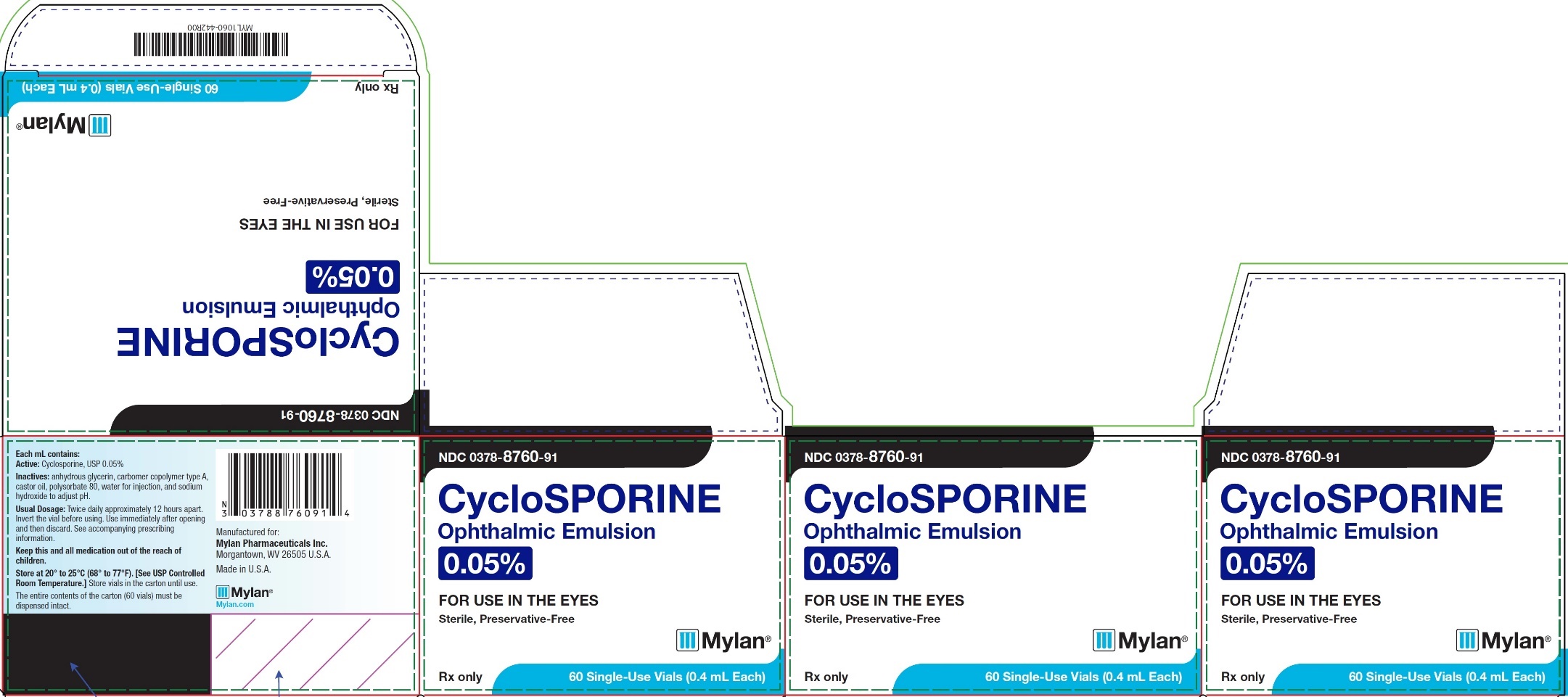
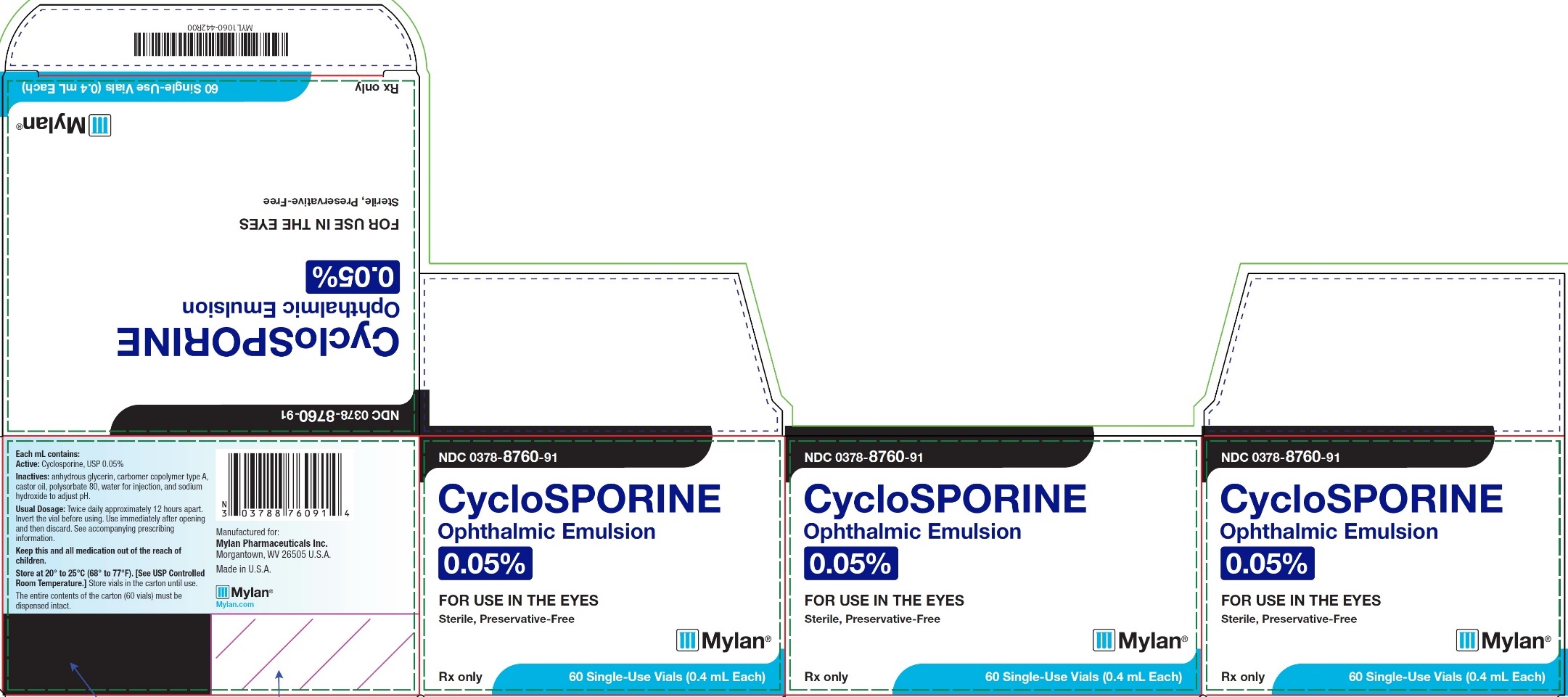
PRINCIPAL DISPLAY PANEL – 0.05% NDC 0378-8760-91 - CycloSPORINE - Ophthalmic Emulsion - 0.05% FOR USE IN THE EYES - Sterile, Preservative-Free - Rx only 60 Single-Use Vials (0.4 mL Each) Each mL contains: Active ...
-
INGREDIENTS AND APPEARANCEProduct Information