Label: METOLAZONE tablet
- NDC Code(s): 0378-6172-01, 0378-6173-01, 0378-6174-01
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDO NOT INTERCHANGE: DO NOT INTERCHANGE ZAROXOLYN®1 TABLETS AND OTHER FORMULATIONS OF METOLAZONE THAT SHARE ITS SLOW AND INCOMPLETE BIOAVAILABILITY AND ARE NOT THERAPEUTICALLY EQUIVALENT AT THE ...
-
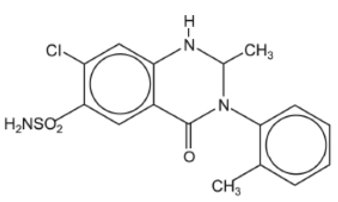
DESCRIPTIONMetolazone Tablets, USP for oral administration contain 2.5 mg, 5 mg or 10 mg of metolazone, USP, a diuretic/saluretic/antihypertensive drug of the quinazoline class. Metolazone has the molecular ...
-
CLINICAL PHARMACOLOGYMetolazone is a quinazoline diuretic, with properties generally similar to the thiazide diuretics. The actions of metolazone result from interference with the renal tubular mechanism of ...
-
INDICATIONS AND USAGEMetolazone tablets are indicated for the treatment of salt and water retention including: • edema accompanying congestive heart failure; • edema accompanying renal diseases, including the ...
-
CONTRAINDICATIONSAnuria, hepatic coma or precoma, known allergy or hypersensitivity to metolazone.
-
WARNINGSRapid Onset Hyponatremia and/or Hypokalemia - Rarely, the rapid onset of severe hyponatremia and/or hypokalemia has been reported following initial doses of thiazide and non-thiazide diuretics ...
-
PRECAUTIONSDO NOT INTERCHANGE: DO NOT INTERCHANGE ZAROXOLYN® TABLETS AND OTHER FORMULATIONS OF METOLAZONE THAT SHARE ITS SLOW AND INCOMPLETE BIOAVAILABILITY AND ARE NOT THERAPEUTICALLY EQUIVALENT AT THE ...
-
ADVERSE REACTIONSMetolazone is usually well tolerated, and most reported adverse reactions have been mild and transient. Many metolazone related adverse reactions represent extensions of its expected pharmacologic ...
-
OVERDOSAGEIntentional overdosage has been reported rarely with metolazone and similar diuretic drugs. Signs and Symptoms - Orthostatic hypotension, dizziness, drowsiness, syncope, electrolyte ...
-
DOSAGE AND ADMINISTRATIONEffective dosage of metolazone tablets should be individualized according to indication and patient response. A single daily dose is recommended. Therapy with metolazone tablets should be titrated ...
-
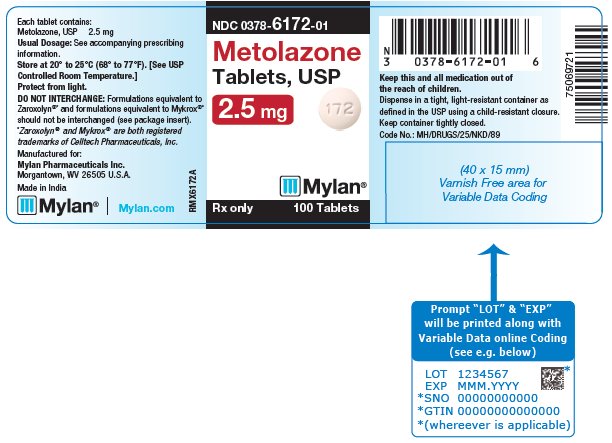
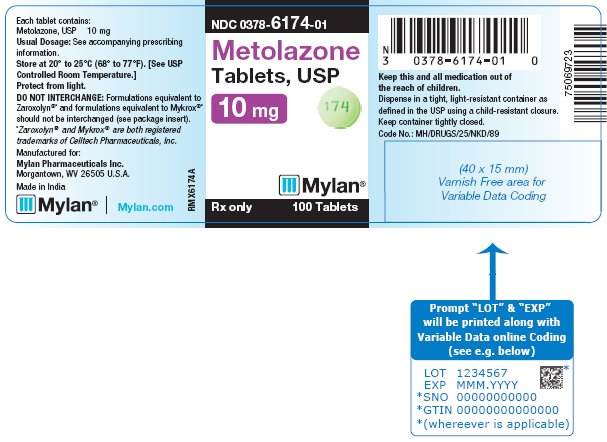
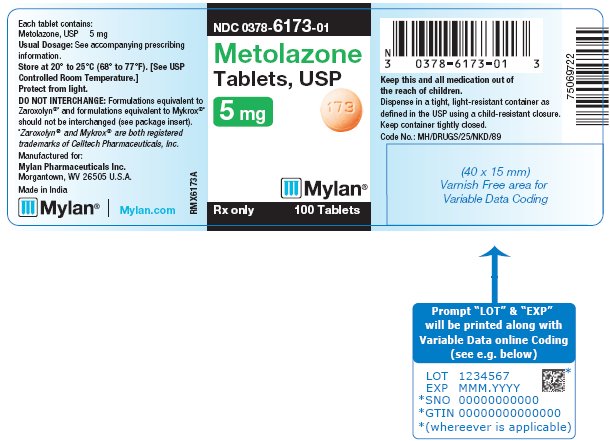
HOW SUPPLIEDMetolazone Tablets, USP are available containing 2.5 mg, 5 mg and 10 mg of metolazone, USP. The 2.5 mg tablets are peach, round, biconvex, unscored tablets debossed with M on one side of the ...
-
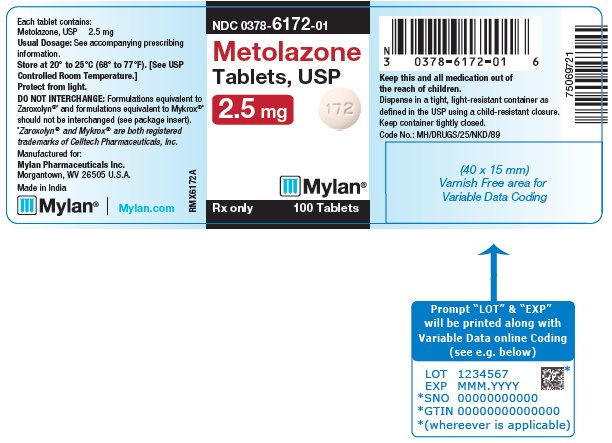
PRINCIPAL DISPLAY PANEL - 2.5 mg NDC 0378-6172-01 - Metolazone - Tablets, USP - 2.5 mg - Rx only 100 Tablets - Each tablet contains: Metolazone, USP 2.5 mg - Usual Dosage: See accompanying - prescribing ...
-
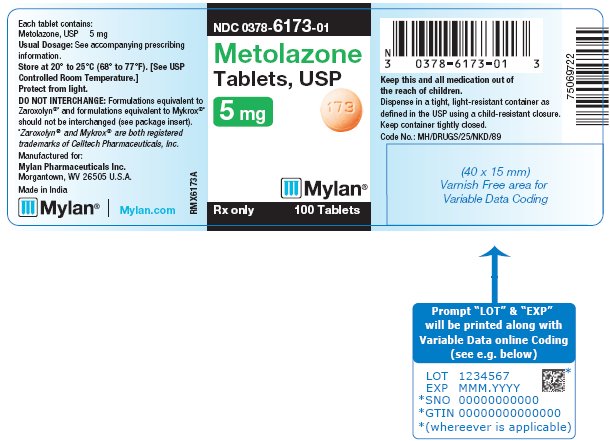
PRINCIPAL DISPLAY PANEL - 5 mgNDC 0378-6173-01 - Metolazone - Tablets, USP - 5 mg - Rx only 100 Tablets - Each tablet contains: Metolazone, USP 5 mg - Usual Dosage: See accompanying - prescribing ...
-
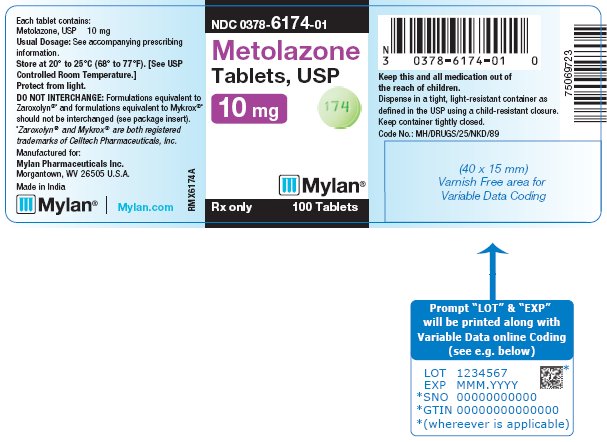
PRINCIPAL DISPLAY PANEL - 10 mg NDC 0378-6174-01 - Metolazone - Tablets, USP - 10 mg - Rx only 100 Tablets - Each tablet contains: Metolazone, USP 10 mg - Usual Dosage: See accompanying - prescribing ...
-
INGREDIENTS AND APPEARANCEProduct Information