Label: METFORMIN HYDROCHLORIDE- metformin tablet, film coated, extended release
- NDC Code(s): 0378-6001-91, 0378-6002-91
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METFORMIN HYDROCHLORIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METFORMIN ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL [see Warnings and Precautions (5.1)].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided [see Dosage and Administration (2.2), Contraindications (4), Warnings and Precautions (5.1)].
If metformin-associated lactic acidosis is suspected, immediately discontinue metformin hydrochloride extended-release tablets and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGE Metformin hydrochloride extended-release tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
-
2 DOSAGE AND ADMINISTRATION 2.1 Adult Dosage and Administration • Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew. • The recommended starting dose of metformin hydrochloride ...
-
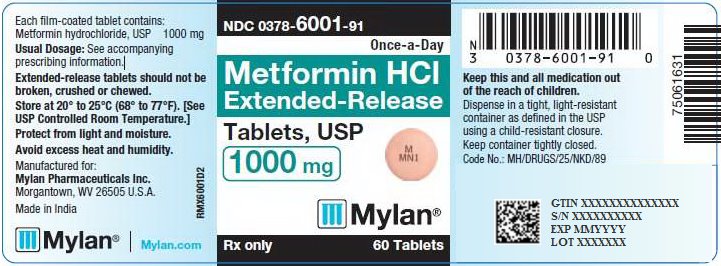
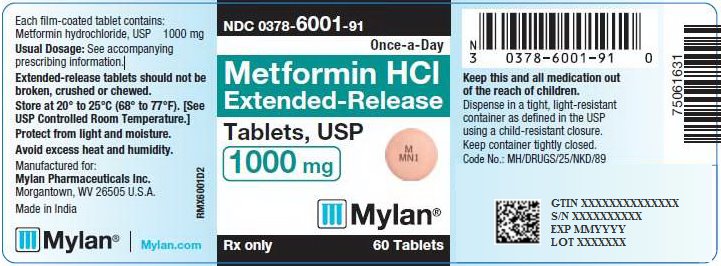
3 DOSAGE FORMS AND STRENGTHS Metformin Hydrochloride Extended-Release Tablets, USP are available containing 500 mg or 1000 mg of metformin hydrochloride, USP. • The 500 mg tablets are pink, film-coated, round, unscored ...
-
4 CONTRAINDICATIONS Metformin hydrochloride extended-release tablets are contraindicated in patients with: • Severe renal impairment (eGFR below 30 mL/min/1.73 m2) [see Warnings and Precautions (5.1)] ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Lactic Acidosis - There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific ...
-
6 ADVERSE REACTIONS The following adverse reactions are also discussed elsewhere in the labeling: • Lactic Acidosis [see Boxed Warning and Warnings and Precautions (5.1)] • Vitamin B12 Deficiency [see Warnings and ...
-
7 DRUG INTERACTIONS Table 2 presents clinically significant drug interactions with metformin hydrochloride extended-release tablets. Table 2: Clinically Significant Drug Interactions with Metformin Hydrochloride ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Limited data with metformin hydrochloride extended-release tablets in pregnant women are not sufficient to determine a drug-associated risk for major birth ...
-
10 OVERDOSAGE Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with ...
-
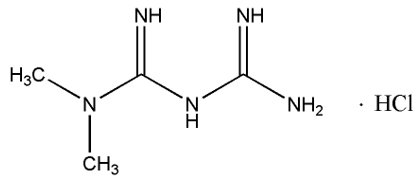
11 DESCRIPTION Metformin hydrochloride extended-release tablets, USP contain the biguanidine antihyperglycemic agent, metformin, in the form of monohydrochloride salt. The chemical name of metformin ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks ...
-
14 CLINICAL STUDIES A 24-week, double-blind, placebo-controlled study of metformin hydrochloride extended-release tablets, taken once daily with the evening meal, was conducted in patients with type 2 diabetes ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - Metformin Hydrochloride Extended-Release Tablets, USP are available containing 500 mg or 1000 mg of metformin hydrochloride, USP. The 500 mg tablets are pink, film-coated ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Lactic Acidosis:Explain the risks of lactic acidosis, its symptoms, and conditions that predispose to its ...
-
Patient Information Metformin Hydrochloride Extended-Release Tablets, USP - (met for’ min hye” droe klor’ ide) What is the most important information I should know about metformin hydrochloride ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 500 mg NDC 0378-6002-91 Once-a-Day - Metformin HCl - Extended-Release - Tablets, USP - 500 mg - Rx only 60 Tablets - Each film-coated tablet contains: Metformin hydrochloride, USP 500 ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 1000 mg NDC 0378-6001-91 Once-a-Day - Metformin HCl - Extended-Release - Tablets, USP - 1000 mg - Rx only 60 Tablets - Each film-coated tablet contains: Metformin hydrochloride, USP 1000 ...
-
INGREDIENTS AND APPEARANCEProduct Information