Label: PRASUGREL tablet, film coated
- NDC Code(s): 0378-5185-93, 0378-5186-93
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRASUGREL TABLETS safely and effectively. See full prescribing information for PRASUGREL TABLETS. PRASUGREL tablets, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: BLEEDING RISK
- •
- Prasugrel tablets can cause significant, sometimes fatal, bleeding [see Warnings and Precautions (5.1, 5.2) and Adverse Reactions (6.1)].
- •
- Do not use prasugrel tablets in patients with active pathological bleeding or a history of transient ischemic attack (TIA) or stroke [see Contraindications (4.1, 4.2)].
- •
- In patients ≥ 75 years of age, prasugrel tablets are generally not recommended, because of the increased risk of fatal and intracranial bleeding and uncertain benefit, except in high-risk situations (patients with diabetes or a history of prior myocardial infarction [MI]) where their effect appears to be greater and their use may be considered [see Use in Specific Populations (8.5)].
- •
- Do not start prasugrel tablets in patients likely to undergo urgent coronary artery bypass graft surgery (CABG). When possible, discontinue prasugrel tablets at least 7 days prior to any surgery [see Warnings and Precautions (5.2)].
- •
- Additional risk factors for bleeding include: body weight < 60 kg, propensity to bleed, concomitant use of medications that increase the risk of bleeding (e.g., warfarin, heparin, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs]) [see Warnings and Precautions (5.1)].
- •
- Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of prasugrel tablets [see Warnings and Precautions (5.1)].
- •
- If possible, manage bleeding without discontinuing prasugrel tablets. Discontinuing prasugrel tablets, particularly in the first few weeks after acute coronary syndrome, increases the risk of subsequent cardiovascular (CV) events [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE 1.1 Acute Coronary Syndrome - Prasugrel tablets are indicated to reduce the rate of thrombotic CV events (including stent thrombosis) in patients with acute coronary syndrome (ACS) who are to be ...
-
2 DOSAGE AND ADMINISTRATION Initiate prasugrel tablets treatment as a single 60 mg oral loading dose and then continue at 10 mg orally once daily. Patients taking prasugrel tablets should also take aspirin (75 mg to 325 mg ...
-
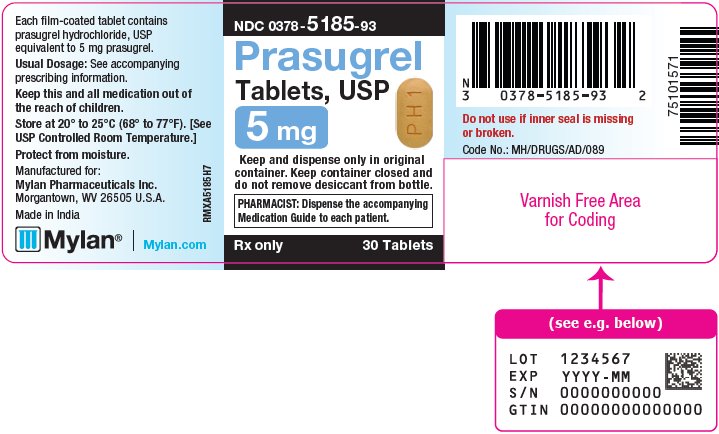
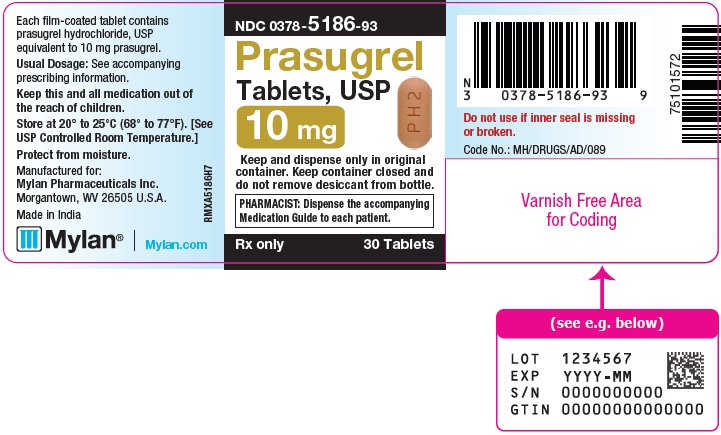
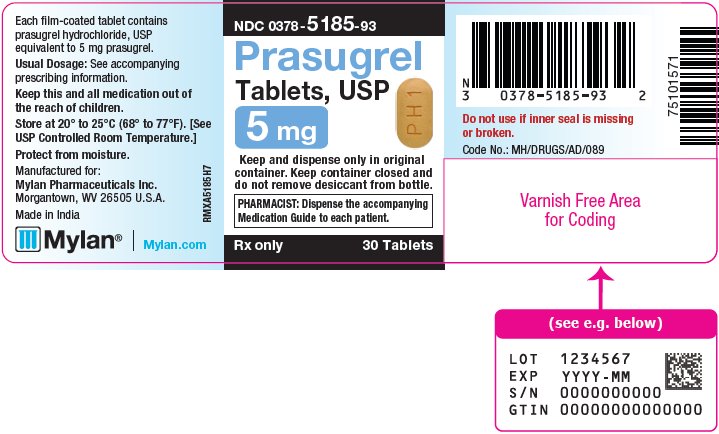
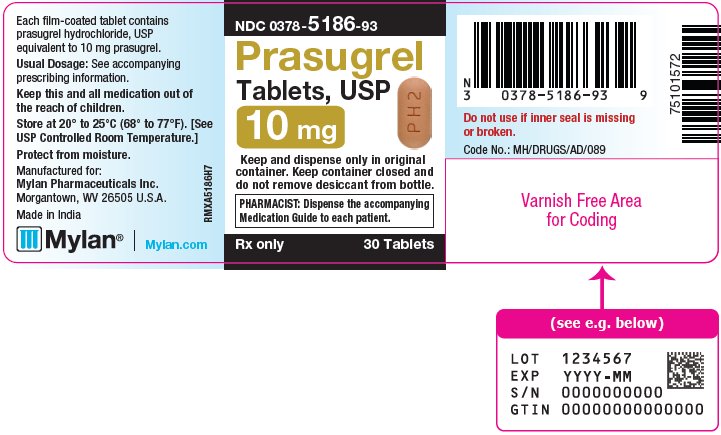
3 DOSAGE FORMS AND STRENGTHS Prasugrel Tablets, USP are available containing prasugrel hydrochloride, USP equivalent to 5 mg or 10 mg prasugrel, respectively. • The 5 mg tablets are yellow, film-coated, capsule shaped ...
-
4 CONTRAINDICATIONS 4.1 Active Bleeding - Prasugrel tablets are contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage (ICH) [see Warnings and Precautions ...
-
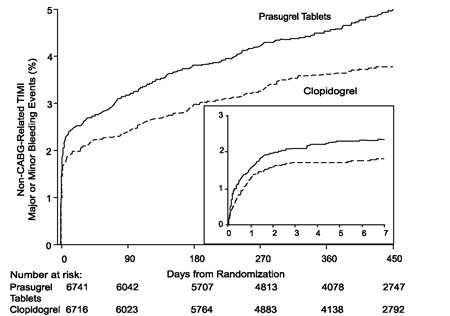
5 WARNINGS AND PRECAUTIONS 5.1 General Risk of Bleeding - Thienopyridines, including prasugrel tablets, increase the risk of bleeding. With the dosing regimens used in TRITON-TIMI 38, TIMI (Thrombolysis in Myocardial ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are also discussed elsewhere in the labeling: • Bleeding [see Boxed Warning and Warnings and Precautions (5.1, 5.2)] • Thrombotic Thrombocytopenic Purpura ...
-
7 DRUG INTERACTIONS 7.1 Warfarin - Coadministration of prasugrel tablets and warfarin increases the risk of bleeding [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]. 7.2 Nonsteroidal ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no data with prasugrel tablets use in pregnant women to inform a drug-associated risk. No structural malformations were observed in animal ...
-
10 OVERDOSAGE 10.1 Signs and Symptoms - Platelet inhibition by prasugrel is rapid and irreversible, lasting for the life of the platelet, and is unlikely to be increased in the event of an overdose. In rats ...
-
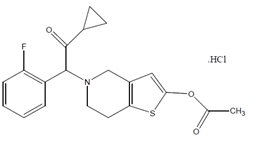
11 DESCRIPTION Prasugrel tablets, USP contain prasugrel, a thienopyridine class inhibitor of platelet activation and aggregation mediated by the P2Y12 ADP receptor. Prasugrel tablets are formulated as the ...
-
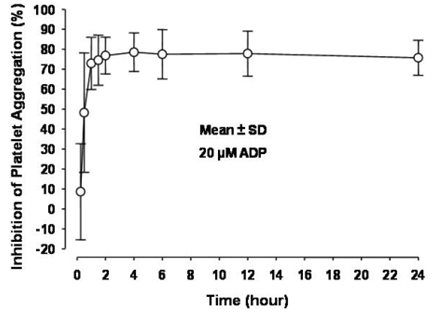
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Prasugrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No compound-related tumors were observed in a 2-year rat study with prasugrel at oral doses up to 100 mg/kg/day ( ...
-
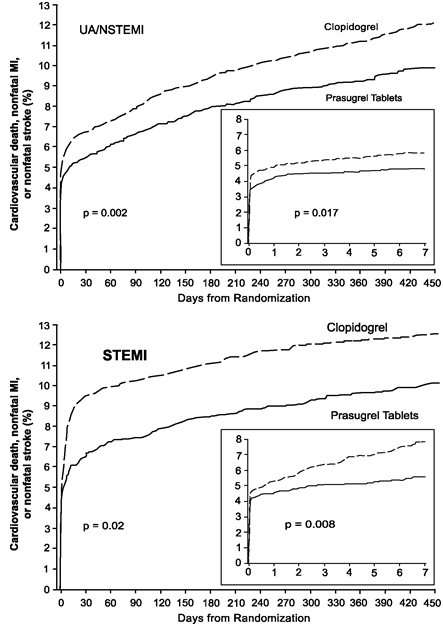
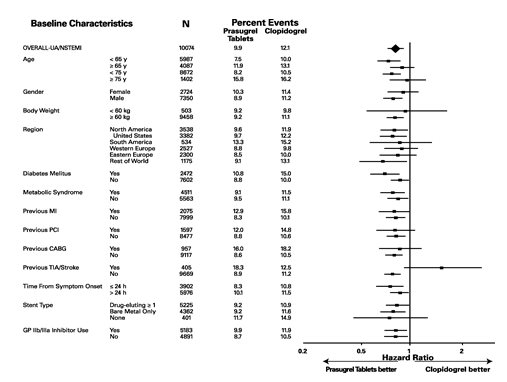
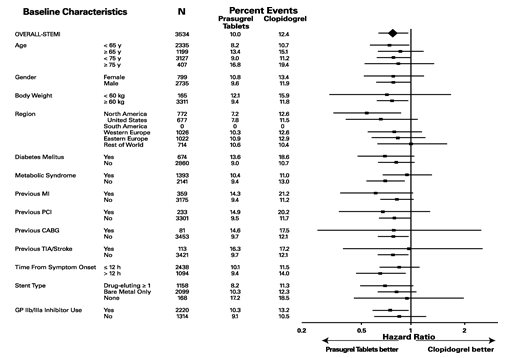
14 CLINICAL STUDIES The clinical evidence for the effectiveness of prasugrel tablets is derived from the TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - Prasugrel Tablets, USP are available containing prasugrel hydrochloride, USP equivalent to 5 mg or 10 mg prasugrel, respectively. The 5 mg tablets are yellow, film-coated ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration: • Advise patients not to break prasugrel tablets. • Remind patients not to discontinue prasugrel ...
-
Medication Guide Prasugrel Tablets, USP - (pra′ soo grel) What is the most important information I should know about prasugrel tablets? Prasugrel tablets are used to lower your chance of having a ...
-
PRINCIPAL DISPLAY PANEL – 5 mg NDC 0378-5185-93 - Prasugrel - Tablets, USP - 5 mg - Keep and dispense only in original - container. Keep container closed and - do not remove desiccant from bottle. PHARMACIST: Dispense the ...
-
PRINCIPAL DISPLAY PANEL – 10 mg NDC 0378-5186-93 - Prasugrel - Tablets, USP - 10 mg - Keep and dispense only in original - container. Keep container closed and - do not remove desiccant from bottle. PHARMACIST: Dispense the ...
-
INGREDIENTS AND APPEARANCEProduct Information