Label: NYSTATIN AND TRIAMCINOLONE ACETONIDE cream
- NDC Code(s): 0316-0223-15, 0316-0223-30, 0316-0223-60
- Packager: Crown Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - For external use only • Not for ophthalmic use.
-
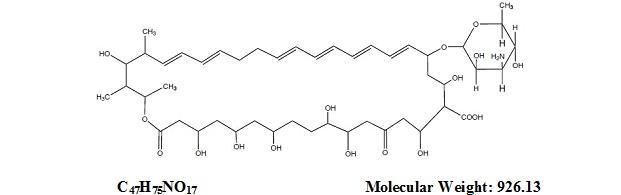
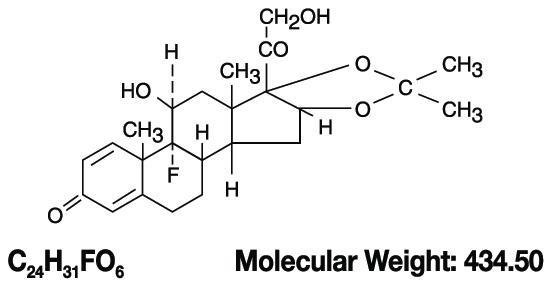
DESCRIPTIONNystatin and Triamcinolone Acetonide Cream for dermatologic use contains the antifungal agent nystatin and the synthetic corticosteroid triamcinolone acetonide. Nystatin is a polyene antimycotic ...
-
CLINICAL PHARMACOLOGYNystatin - Nystatin exerts its antifungal activity against a variety of pathogenic and nonpathogenic yeasts and fungi by binding to sterols in the cell membrane. The binding process renders the ...
-
INDICATIONS AND USAGENystatin and Triamcinolone Acetonide Cream is indicated for the treatment of cutaneous candidiasis; it has been demonstrated that the nystatin-steroid combination provides greater benefit than the ...
-
CONTRAINDICATIONSThese preparations are contraindicated in those patients with a history of hypersensitivity to any of their components.
-
PRECAUTIONSGeneral - Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and ...
-
Information for the PatientPatients using this medication should receive the following information and instructions: This medication is to be used as directed by the physician. It is for external use only. Avoid contact ...
-
Laboratory TestsIf there is a lack of therapeutic response, appropriate microbiological studies (e.g. KOH smears and/or cultures) should be repeated to confirm the diagnosis and rule out other pathogens, before ...
-
Carcinogenesis, Mutagenesis, and Impairment of FertilityLong-term animal studies have not been performed to evaluate carcinogenic or mutagenic potential, or possible impairment of fertility in males or females.
-
Pregnancy Category CThere are no teratogenic studies with combined nystatin and triamcinolone acetonide. Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively ...
-
Nursing MothersIt is not known whether any component of this preparation is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised during the use of this preparation ...
-
Pediatric UseIn clinical studies of a limited number of pediatric patients ranging from two months through 12 years, nystatin and triamcinolone acetonide cream formulation cleared or significantly ameliorated ...
-
ADVERSE REACTIONSA single case (approximately one percent of patients studied) of acneiform eruption occurred with use of combined nystatin and triamcinolone acetonide in clinical studies. Nystatin is virtually ...
-
OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see - PRECAUTIONS, General); however, acute overdosage and serious adverse effects ...
-
DOSAGE AND ADMINISTRATIONNystatin and Triamcinolone Acetonide Cream is usually applied to the affected areas twice daily in the morning and evening by gently and thoroughly massaging the preparation into the skin. The ...
-
HOW SUPPLIEDNystatin and Triamcinolone Acetonide Cream is supplied in: 15 gram tube NDC 0316-0223-15 - 30 gram tube NDC 0316-0223-30 - 60 gram tube NDC 0316-0223-60
-
STORAGE AND HANDLINGStore at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Avoid freezing. Manufactured and Distributed by: Crown Laboratories, Inc. Johnson City, TN 37604 - REVISED: OCT ...
-
Nystatin and Triamcinolone Acetonide Cream 30 gram tubeNDC 0316-0223-30 - Nystatin and Triamcinolone Acetonide Cream USP - 30 grams - For external use only. Not for ophthalmic use. Rx Only - P9508.01
-
Nystatin and Triamcinolone Acetonide Cream 30 gram cartonNDC 0316-0223-30 - Nystatin and Triamcinolone Acetonide Cream USP - 30 grams - For external use only. Not for ophthalmic use. Rx Only - P9520.02
-
INGREDIENTS AND APPEARANCEProduct Information