Label: VANDAZOLE- metronidazole gel
- NDC Code(s): 0245-0860-70
- Packager: Upsher-Smith Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VANDAZOLE® safely and effectively. See full prescribing information for VANDAZOLE. VANDAZOLE® (metronidazole gel), for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVANDAZOLE® is indicated in the treatment of bacterial vaginosis (formerly referred to as Haemophilus vaginitis, Gardnerella vaginitis, nonspecific vaginitis, Corynebacterium vaginitis, or ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose is one applicator full of VANDAZOLE, (approximately 5 grams of gel containing approximately 37.5 mg of metronidazole) administered intravaginally once a day for 5 days. For ...
-
3 DOSAGE FORMS AND STRENGTHSGel, 0.75%. VANDAZOLE is a clear, colorless to yellow gel in a 70 g tube, supplied with 5 vaginal applicators. Each applicator delivers approximately 5 g of gel containing 37.5 mg of ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - The use of VANDAZOLE is contraindicated in patients with a history of hypersensitivity to metronidazole, other nitroimidazole derivatives, or parabens. Reported reactions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Central and Peripheral Nervous System Effects - Use of oral or intravenous metronidazole is associated with convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSThe intravaginal administration of a single 5 gram dose of VANDAZOLE results in relatively lower mean systemic exposure to metronidazole that is approximately 2% to 5% of that achieved following a ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data on metronidazole use in pregnant women from published cohort studies, case-control studies, case series, meta analyses, and case reports over several ...
-
10 OVERDOSAGEThere is no human experience with overdosage of metronidazole vaginal gel. Vaginally applied metronidazole gel, 0.75% could be absorbed in sufficient amounts to produce systemic effects [see ...
-
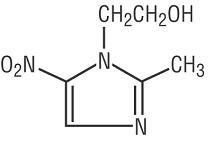
11 DESCRIPTIONVANDAZOLE (metronidazole gel, USP), 0.75% is the vaginal dosage form of the nitroimidazole antimicrobial metronidazole at a concentration of 0.75%. Chemically, metronidazole is a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metronidazole is an antibacterial drug [see Clinical Pharmacology, Microbiology (12.4)] 12.3 Pharmacokinetics - Healthy Subjects - Following a single, intravaginal 5 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Metronidazole has shown evidence of carcinogenic activity after chronic oral administration in mice and rats. Pulmonary tumors and ...
-
14 CLINICAL STUDIESA single, randomized, double-blind, active-controlled clinical trial was conducted to evaluate the efficacy of VANDAZOLE for the treatment of bacterial vaginosis. A clinical diagnosis of bacterial ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVANDAZOLE (metronidazole gel, USP), 0.75 % is supplied in a 70-gram tube and is packaged with 5 vaginal applicators. (NDC 0245-0860-70) Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Interaction with Alcohol - Instruct the patient not to consume alcoholic beverages and preparations ...
-
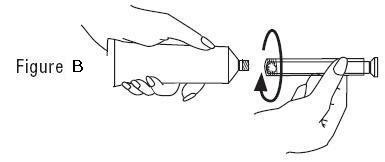
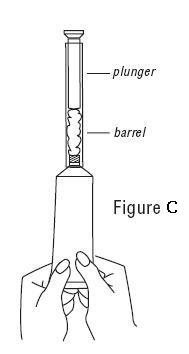
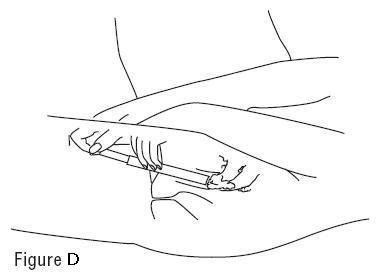
PATIENT INSTRUCTIONS FOR USEVANDAZOLE® (van-DA-zole) (metronidazole gel, USP), 0.75% For vaginal use only. Do not put VANDAZOLE in your eyes, mouth, or on your skin. Read this Patient Instructions for Use before you ...
-
Package/Label Display PanelNDC 0245-0860-70 - VANDAZOLE® (Metronidazole Gel USP, 0.75% [Vaginal]) with 5 applicators - FOR INTRAVAGINAL USE ONLY. (NOT FOR OPHTHAMIC, DERMAL, OR ORAL USE.) Rx only - Net Wt. 70 ...
-
INGREDIENTS AND APPEARANCEProduct Information