Mechanism of Action
The mechanism of the antihypertensive effect of propranolol has not been established. Among the factors that may be involved in contributing to the antihypertensive action include: (1) decreased cardiac output, (2) inhibition of renin release by the kidneys, and (3) diminution of tonic sympathetic nerve outflow from vasomotor centers in the brain. Although total peripheral resistance may increase initially, it readjusts to or below the pretreatment level with chronic use of propranolol. Effects of propranolol on plasma volume appear to be minor and somewhat variable.
In angina pectoris, propranolol generally reduces the oxygen requirement of the heart at any given level of effort by blocking the catecholamine-induced increases in the heart rate, systolic blood pressure, and the velocity and extent of myocardial contraction. Propranolol may increase oxygen requirements by increasing left ventricular fiber length, end diastolic pressure, and systolic ejection period. The net physiologic effect of beta-adrenergic blockade is usually advantageous and is manifested during exercise by delayed onset of pain and increased work capacity.
Propranolol exerts its antiarrhythmic effects in concentrations associated with beta-adrenergic blockade, and this appears to be its principal antiarrhythmic mechanism of action. In dosages greater than required for beta blockade, propranolol also exerts a quinidine-like or anesthetic-like membrane action which affects the cardiac action potential. The significance of the membrane action in the treatment of arrhythmias is uncertain.
The mechanism of the anti-migraine effect of propranolol has not been established. Beta-adrenergic receptors have been demonstrated in the pial vessels of the brain.
PHARMACOKINETICS AND DRUG METABOLISM
Absorption
Propranolol is highly lipophilic and almost completely absorbed after oral administration. However, it undergoes high first pass metabolism by the liver and on average, only about 25% of propranolol reaches the systemic circulation. Propranolol hydrochloride extended-release capsules (60 mg, 80 mg, 120 mg, and 160 mg) release propranolol hydrochloride at a controlled and predictable rate. Peak blood levels following dosing with propranolol hydrochloride extended-release capsules occur at about 6 hours.
The effect of food on propranolol hydrochloride extended-release capsules bioavailability has not been investigated.
Distribution
Approximately 90% of circulating propranolol is bound to plasma proteins (albumin and alpha-1-acid glycoprotein). The binding is enantiomer-selective. The S(–)-enantiomer is preferentially bound to alpha-1-glycoprotein and the R(+)-enantiomer preferentially bound to albumin. The volume of distribution of propranolol is approximately 4 liters/kg.
Propranolol crosses the blood-brain barrier and the placenta, and is distributed into breast milk.
Metabolism and Elimination
Propranolol is extensively metabolized with most metabolites appearing in the urine. Propranolol is metabolized through three primary routes: aromatic hydroxylation (mainly 4-hydroxylation), N-dealkylation followed by further side-chain oxidation, and direct glucuronidation. It has been estimated that the percentage contributions of these routes to total metabolism are 42%, 41% and 17%, respectively, but with considerable variability between individuals. The four major metabolites are propranolol glucuronide, naphthyloxylactic acid and glucuronic acid, and sulfate conjugates of 4-hydroxy propranolol.
In vitro studies have indicated that the aromatic hydroxylation of propranolol is catalyzed mainly by polymorphic CYP2D6. Side-chain oxidation is mediated mainly by CYP1A2 and to some extent by CYP2D6. 4-hydroxy propranolol is a weak inhibitor of CYP2D6.
Propranolol is also a substrate of CYP2C19 and a substrate for the intestinal efflux transporter, p-glycoprotein (p-gp). Studies suggest however that p-gp is not dose-limiting for intestinal absorption of propranolol in the usual therapeutic dose range.
In healthy subjects, no difference was observed between CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs) with respect to oral clearance or elimination half-life. Partial clearance of 4-hydroxy propranolol was significantly higher and naphthyloxylactic acid was significantly lower in EMs than PMs.
When measured at steady state over a 24-hour period the areas under the propranolol plasma concentration-time curve (AUCs) for the propranolol hydrochloride extended-release capsules are approximately 60% to 65% of the AUCs for a comparable divided daily dose of propranolol hydrochloride extended-release tablets. The lower AUCs for the propranolol hydrochloride extended-release capsules are due to greater hepatic metabolism of propranolol, resulting from the slower rate of absorption of propranolol. Over a twenty-four (24) hour period, blood levels are fairly constant for about twelve (12) hours, then decline exponentially. The apparent plasma half-life is about 10 hours.
Enantiomers
Propranolol is a racemic mixture of two enantiomers, R(+) and S(–). The S(–)-enantiomer is approximately 100 times as potent as the R(+)-enantiomer in blocking beta-adrenergic receptors. In normal subjects receiving oral doses of racemic propranolol, S(–)-enantiomer concentrations exceeded those of the R(+)-enantiomer by 40 to 90% as a result of stereoselective hepatic metabolism. Clearance of the pharmacologically active S(–)-propranolol is lower than R(+)-propranolol after intravenous and oral doses.
Special Population
Geriatric
The pharmacokinetics of propranolol hydrochloride extended-release capsules have not been investigated in patients over 65 years of age. In a study of 12 elderly (62 to 79 years old) and 12 young (25 to 33 years old) healthy subjects, the clearance of S-enantiomer of propranolol was decreased in the elderly. Additionally, the half-life of both the R- and S-propranolol were prolonged in the elderly compared with the young (11 hours vs. 5 hours).
Clearance of propranolol is reduced with aging due to decline in oxidation capacity (ring oxidation and side chain oxidation). Conjugation capacity remains unchanged. In a study of 32 patients age 30 to 84 years given a single 20 mg dose of propranolol, an inverse correlation was found between age and the partial metabolic clearances to 4-hydroxypropranolol (40HP ring oxidation) and to naphthoxylactic acid (NLA-side chain oxidation). No correlation was found between age and the partial metabolic clearance to propranolol glucuronide (PPLG conjugation).
Gender
In a study of 9 healthy women and 12 healthy men, neither the administration of testosterone nor the regular course of the menstrual cycle affected the plasma binding of the propranolol enantiomers. In contrast, there was a significant, although non-enantioselective diminution of the binding of propranolol after treatment with ethinyl estradiol. These findings are inconsistent with another study, in which administration of testosterone cypionate confirmed the stimulatory role of this hormone on propranolol metabolism and concluded that the clearance of propranolol in men is dependent on circulating concentrations of testosterone. In women, none of the metabolic clearances for propranolol showed any significant association with either estradiol or testosterone.
Race
A study conducted in 12 Caucasian and 13 African-American male subjects taking propranolol, showed that at steady state, the clearance of R(+)- and S(–)-propranolol were about 76% and 53% higher in African-Americans than in Caucasians, respectively.
Chinese subjects had a greater proportion (18% to 45% higher) of unbound propranolol in plasma compared to Caucasians, which was associated with a lower plasma concentration of alpha-1-acid glycoprotein.
Renal Insufficiency
The pharmacokinetics of propranolol hydrochloride extended-release capsules have not been investigated in patients with renal insufficiency.
In a study conducted in 5 patients with chronic renal failure, 6 patients on regular dialysis, and 5 healthy subjects, who received a single oral dose of 40 mg of propranolol, the peak plasma concentrations (Cmax) of propranolol in the chronic renal failure group were 2 to 3-fold higher (161±41 ng/mL) than those observed in the dialysis patients (47±9 ng/mL) and in the healthy subjects (26±1 ng/mL). Propranolol plasma clearance was also reduced in the patients with chronic renal failure.
Studies have reported a delayed absorption rate and a reduced half-life of propranolol in patients with renal failure of varying severity. Despite this shorter plasma half-life, propranolol peak plasma levels were 3 to 4 times higher and total plasma levels of metabolites were up to 3 times higher in these patients than in subjects with normal renal function.
Chronic renal failure has been associated with a decrease in drug metabolism via down regulation of hepatic cytochrome P450 activity resulting in a lower “first-pass” clearance.
Propranolol is not significantly dialyzable.
Hepatic Insufficiency
The pharmacokinetics of propranolol hydrochloride extended-release capsules have not been investigated in patients with hepatic insufficiency.
Propranolol is extensively metabolized by the liver. In a study conducted in 6 patients with cirrhosis and 7 healthy subjects receiving 160 mg of a long-acting preparation of propranolol once a day for 7 days, the steady-state propranolol concentration in patients with cirrhosis was increased 2.5-fold in comparison to controls. In the patients with cirrhosis, the half-life obtained after a single intravenous dose of 10 mg propranolol increased to 7.2 hours compared to 2.9 hours in control (see PRECAUTIONS).
Drug Interactions
All drug interaction studies were conducted with propranolol. There are no data on drug interactions with propranolol hydrochloride extended-release capsules.
Interactions with Substrates, Inhibitors or Inducers of Cytochrome P-450 Enzymes
Because propranolol’s metabolism involves multiple pathways in the Cytochrome P-450 system (CYP2D6, 1A2, 2C19), coadministration with drugs that are metabolized by, or affect the activity (induction or inhibition) of one or more of these pathways may lead to clinically relevant drug interactions (see Drug Interactions under PRECAUTIONS).
Substrates or Inhibitors of CYP2D6
Blood levels and/or toxicity of propranolol may be increased by coadministration with substrates or inhibitors of CYP2D6, such as amiodarone, cimetidine, delavudin, fluoxetine, paroxetine, quinidine, and ritonavir. No interactions were observed with either ranitidine or lansoprazole.
Substrates or Inhibitors of CYP1A2
Blood levels and/or toxicity of propranolol may be increased by coadministration with substrates or inhibitors of CYP1A2, such as imipramine, cimetidine, ciprofloxacin, fluvoxamine, isoniazid, ritonavir, theophylline, zileuton, zolmitriptan, and rizatriptan.
Substrates or Inhibitors of CYP2C19
Blood levels and/or toxicity of propranolol may be increased by coadministration with substrates or inhibitors of CYP2C19, such as fluconazole, cimetidine, fluoxetine, fluvoxamine, tenioposide, and tolbutamide. No interaction was observed with omeprazole.
Inducers of Hepatic Drug Metabolism
Blood levels of propranolol may be decreased by coadministration with inducers such as rifampin, ethanol, phenytoin, and phenobarbital. Cigarette smoking also induces hepatic metabolism and has been shown to increase up to 77% the clearance of propranolol, resulting in decreased plasma concentrations.
Cardiovascular Drugs
Antiarrhythmics
The AUC of propafenone is increased by more than 200% by coadministration of propranolol.
The metabolism of propranolol is reduced by coadministration of quinidine, leading to a two to three fold increased blood concentration and greater degrees of clinical beta-blockade.
The metabolism of lidocaine is inhibited by coadministration of propranolol, resulting in a 25% increase in lidocaine concentrations.
Calcium Channel Blockers
The mean Cmax and AUC of propranolol are increased respectively, by 50% and 30% by coadministration of nisoldipine and by 80% and 47%, by coadministration of nicardipine.
The mean Cmax and AUC of nifedipine are increased by 64% and 79%, respectively, by coadministration of propranolol.
Propranolol does not affect the pharmacokinetics of verapamil and norverapamil. Verapamil does not affect the pharmacokinetics of propranolol.
Non-Cardiovascular Drugs
Migraine Drugs
Administration of zolmitriptan or rizatriptan with propranolol resulted in increased concentrations of zolmitriptan (AUC increased by 56% and Cmax by 37%) or rizatriptan (the AUC and Cmax were increased by 67% and 75%, respectively).
Theophylline
Coadministration of theophylline with propranolol decreases theophylline oral clearance by 30% to 52%.
Benzodiazepines
Propranolol can inhibit the metabolism of diazepam, resulting in increased concentrations of diazepam and its metabolites. Diazepam does not alter the pharmacokinetics of propranolol.
The pharmacokinetics of oxazepam, triazolam, lorazepam, and alprazolam are not affected by coadministration of propranolol.
Neuroleptic Drugs
Coadministration of long-acting propranolol at doses greater than or equal to 160 mg/day resulted in increased thioridazine plasma concentrations ranging from 55% to 369% and increased thioridazine metabolite (mesoridazine) concentrations ranging from 33% to 209%.
Coadministration of chlorpromazine with propranolol resulted in a 70% increase in propranolol plasma level.
Anti-Ulcer Drugs
Coadministration of propranolol with cimetidine, a non-specific CYP450 inhibitor, increased propranolol AUC and Cmax by 46% and 35%, respectively. Coadministration with aluminum hydroxide gel (1,200 mg) may result in a decrease in propranolol concentrations.
Coadministration of metoclopramide with the long-acting propranolol did not have a significant effect on propranolol’s pharmacokinetics.
Lipid Lowering Drugs
Coadministration of cholestyramine or colestipol with propranolol resulted in up to 50% decrease in propranolol concentrations.
Coadministration of propranolol with lovastatin or pravastatin, decreased 18% to 23% the AUC of both, but did not alter their pharmacodynamics. Propranolol did not have an effect on the pharmacokinetics of fluvastatin.
Warfarin
Concomitant administration of propranolol and warfarin has been shown to increase warfarin bioavailability and increase prothrombin time.
PHARMACODYNAMICS AND CLINICAL EFFECTS
Hypertension
In a retrospective, uncontrolled study, 107 patients with diastolic blood pressure 110 to 150 mmHg received propranolol 120 mg t.i.d. for at least 6 months, in addition to diuretics and potassium, but with no other hypertensive agent. Propranolol contributed to control of diastolic blood pressure, but the magnitude of the effect of propranolol on blood pressure cannot be ascertained.
Four double-blind, randomized, crossover studies were conducted in a total of 74 patients with mild or moderately severe hypertension treated with propranolol hydrochloride extended-release capsules 160 mg once daily or propranolol 160 mg given either once daily or in two 80 mg doses. Three of these studies were conducted over a 4-week treatment period. One study was assessed after a 24-hour period. Propranolol hydrochloride extended-release capsules were as effective as propranolol in controlling hypertension (pulse rate, systolic and diastolic blood pressure) in each of these trials.
Angina Pectoris
In a double-blind, placebo-controlled study of 32 patients of both sexes, aged 32 to 69 years, with stable angina, propranolol 100 mg t.i.d. was administered for 4 weeks and shown to be more effective than placebo in reducing the rate of angina episodes and in prolonging total exercise time.
Twelve male patients with moderately severe angina pectoris were studied in a double-blind, crossover study. Patients were randomized to either propranolol hydrochloride extended-release capsules 160 mg daily or conventional propranolol 40 mg four times a day for 2 weeks. Nitroglycerine tablets were allowed during the study. Blood pressure, heart rate and ECG's were recorded during serial exercise treadmill testing. Propranolol hydrochloride extended-release capsules were as effective as conventional propranolol for exercise heart rate, systolic and diastolic blood pressure, duration of anginal pain and ST-segment depression before or after exercise, exercise duration, angina attack rate and nitroglycerine consumption.
In another double-blind, randomized, crossover trial, the effectiveness of propranolol hydrochloride extended-release capsules 160 mg daily and conventional propranolol 40 mg four times a day were evaluated in 13 patients with angina. ECG's were recorded while patients exercised until angina developed. Propranolol hydrochloride extended-release capsules were as effective as conventional propranolol for amount of exercise performed, ST-segment depression, number of anginal attacks, amount of nitroglycerine consumed, systolic and diastolic blood pressures and heart rate at rest and after exercise.
Migraine
In a 34-week, placebo-controlled, 4-period, dose-finding crossover study with a double-blind randomized treatment sequence, 62 patients with migraine received propranolol 20 to 80 mg 3 or 4 times daily. The headache unit index, a composite of the number of days with headache and the associated severity of the headache, was significantly reduced for patients receiving propranolol as compared to those on placebo.
Hypertrophic Subaortic Stenosis
In an uncontrolled series of 13 patients with New York Heart Association (NYHA) class 2 or 3 symptoms and hypertrophic subaortic stenosis diagnosed at cardiac catheterization, oral propranolol 40 to 80 mg t.i.d. was administered and patients were followed for up to 17 months.
Propranolol was associated with improved NYHA class for most patients.



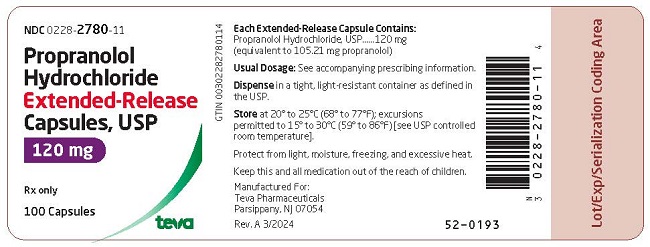

 and 2778 on the cap and body in black ink contains 60 mg of propranolol hydrochloride, USP (equivalent to 52.60 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2778-11).
and 2778 on the cap and body in black ink contains 60 mg of propranolol hydrochloride, USP (equivalent to 52.60 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2778-11).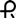 and 2779 on the cap and body in black ink contains 80 mg of propranolol hydrochloride, USP (equivalent to 70.14 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2779-11).
and 2779 on the cap and body in black ink contains 80 mg of propranolol hydrochloride, USP (equivalent to 70.14 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2779-11). and 2780 on the cap and body in black ink contains 120 mg of propranolol hydrochloride, USP (equivalent to 105.21 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2780-11).
and 2780 on the cap and body in black ink contains 120 mg of propranolol hydrochloride, USP (equivalent to 105.21 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2780-11). and 2781 on the cap and body in black ink contains 160 mg of propranolol hydrochloride, USP (equivalent to 140.28 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2781-11).
and 2781 on the cap and body in black ink contains 160 mg of propranolol hydrochloride, USP (equivalent to 140.28 mg of propranolol). Capsules are supplied in bottles of 100 (NDC 0228-2781-11).


