Label: ISOSORBIDE MONONITRATE tablet
- NDC Code(s): 0228-2620-11, 0228-2631-11
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Isosorbide mononitrate (diluted), USP, an organic nitrate, is a vasodilator with effects on both arteries and veins. The molecular formula is C6H9NO6 and the molecular weight is 191.14. The ...
-
CLINICAL PHARMACOLOGY
Isosorbide mononitrate is the major active metabolite of isosorbide dinitrate (ISDN), and most of the clinical activity of the dinitrate is attributable to the mononitrate. The principal ...
-
INDICATIONS AND USAGE
Isosorbide mononitrate tablets, USP are indicated for the prevention and treatment of angina pectoris due to coronary artery disease. The onset of action of oral isosorbide mononitrate is not ...
-
CONTRAINDICATIONS
Isosorbide mononitrate is contraindicated in patients who are allergic to it. Do not use isosorbide mononitrate in patients who are taking certain drugs for erectile dysfunction (phosphodiesterase ...
-
WARNINGS
Amplification of the vasodilatory effects of isosorbide mononitrate by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied ...
-
PRECAUTIONS
General - Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide mononitrate. This drug should therefore be used with caution in patients who may ...
-
ADVERSE REACTIONS
Headache is the most frequent side effect and was the cause of 2% of all dropouts from controlled-clinical trials. Headache decreased in incidence after the first few days of therapy. The ...
-
OVERDOSAGE
Hemodynamic Effects - The ill effects of isosorbide mononitrate overdose are generally the results of isosorbide mononitrate’s capacity to induce vasodilatation, venous pooling, reduced cardiac ...
-
DOSAGE AND ADMINISTRATION
The recommended regimen of isosorbide mononitrate tablets is 20 mg twice daily, with the doses seven hours apart. A starting dose of 5 mg (½ tablet of the 10 mg dosing strength) might be ...
-
HOW SUPPLIED
Isosorbide mononitrate tablets, USP are available as follows: 10 mg — Each blue, round, tablet imprinted with and 631 on one side and scored on the other side, contains 10 mg of isosorbide ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0228-2631-11 - Isosorbide Mononitrate Tablets, USP - 10 mg - 100 Tablets - Rx only
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0228-2620-11 - Isosorbide Mononitrate Tablets, USP - 20 mg - 100 Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information



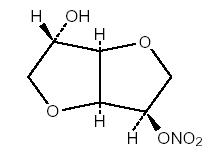
 and 631 on one side and scored on the other side, contains 10 mg of isosorbide mononitrate (diluted), USP. Tablets are supplied in bottles of 100 (NDC 0228-2631-11).
and 631 on one side and scored on the other side, contains 10 mg of isosorbide mononitrate (diluted), USP. Tablets are supplied in bottles of 100 (NDC 0228-2631-11). and 620 on one side and scored on the other side, contains 20 mg of isosorbide mononitrate (diluted), USP. Tablets are supplied in bottles of 100 (NDC 0228-2620-11).
and 620 on one side and scored on the other side, contains 20 mg of isosorbide mononitrate (diluted), USP. Tablets are supplied in bottles of 100 (NDC 0228-2620-11).
