Label: INDAPAMIDE tablet, film coated
- NDC Code(s): 0228-2571-11, 0228-2571-96, 0228-2597-11, 0228-2597-96
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
DESCRIPTION
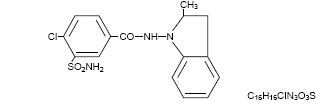
Indapamide is an oral antihypertensive/diuretic. Its molecule contains both a polar sulfamoyl chlorobenzamide moiety and a lipid-soluble methylindoline moiety. It differs chemically from the ...
-
CLINICAL PHARMACOLOGY
Indapamide is the first of a new class of antihypertensive/diuretics, the indolines. The oral administration of 2.5 mg (two 1.25 mg tablets) of indapamide to male subjects produced peak ...
-
INDICATIONS AND USAGE
Indapamide tablets are indicated for the treatment of hypertension, alone or in combination with other antihypertensive drugs. Indapamide tablets are also indicated for the treatment of salt and ...
-
CONTRAINDICATIONS
Anuria. Known hypersensitivity to indapamide or to other sulfonamide-derived drugs.
-
WARNINGS
Severe cases of hyponatremia, accompanied by hypokalemia have been reported with recommended doses of indapamide. This occurred primarily in elderly females (see PRECAUTIONS, Geriatric Use). This ...
-
PRECAUTIONS
General: Hypokalemia, Hyponatremia, and Other Fluid and Electrolyte Imbalances: Periodic determinations of serum electrolytes should be performed at appropriate intervals. In addition ...
-
ADVERSE REACTIONS
Most adverse effects have been mild and transient. The Clinical Adverse Reactions listed in Table 1 represent data from Phase II/III placebo-controlled studies (306 patients given indapamide 1.25 ...
-
OVERDOSAGE
Symptoms of overdosage include nausea, vomiting, weakness, gastrointestinal disorders and disturbances of electrolyte balance. In severe instances, hypotension and depressed respiration may be ...
-
DOSAGE AND ADMINISTRATION
Hypertension: The adult starting indapamide dose for hypertension is 1.25 mg as a single daily dose taken in the morning. If the response to 1.25 mg is not satisfactory after four weeks, the ...
-
HOW SUPPLIED
Indapamide Tablets, USP are available as follows: 1.25 mg — Each orange, round, film-coated tablet imprinted with on one side and 597 on the other side contains 1.25 mg of indapamide, USP ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0228-2597-11 - Indapamide Tablets, USP 1.25 mg - Rx only - 100 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 0228-2571-11 - Indapamide Tablets, USP 2.5 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information



 on one side and 597 on the other side contains 1.25 mg of indapamide, USP. Tablets are supplied in bottles of 100 (NDC 0228-2597-11) and 1000 (NDC 0228-2597-96).
on one side and 597 on the other side contains 1.25 mg of indapamide, USP. Tablets are supplied in bottles of 100 (NDC 0228-2597-11) and 1000 (NDC 0228-2597-96). on one side and 571 on the other side contains 2.5 mg of indapamide, USP. Tablets are supplied in bottles of 100 (NDC 0228-2571-11) and 1000 (NDC 0228-2571-96).
on one side and 571 on the other side contains 2.5 mg of indapamide, USP. Tablets are supplied in bottles of 100 (NDC 0228-2571-11) and 1000 (NDC 0228-2571-96).
