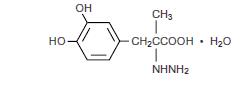
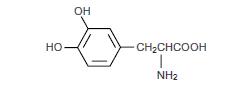
General
-
As with levodopa, periodic evaluations of hepatic, hematopoietic, cardiovascular, and renal function are recommended during extended therapy.
Patients with chronic wide-angle glaucoma ...
General
As with levodopa, periodic evaluations of hepatic, hematopoietic, cardiovascular, and renal function are recommended during extended therapy.
Patients with chronic wide-angle glaucoma may be treated cautiously with carbidopa and levodopa provided the intraocular pressure is well-controlled and the patient is monitored carefully for changes in intraocular pressure during therapy.
Dyskinesia
Levodopa alone, as well as carbidopa and levodopa, is associated with dyskinesias. The occurrence of dyskinesias may require dosage reduction.
Hallucinations/Psychotic-Like Behavior
Hallucinations and psychotic-like behavior have been reported with dopaminergic medications. In general, hallucinations present shortly after the initiation of therapy and may be responsive to dose reduction in levodopa. Hallucinations may be accompanied by confusion and to a lesser extent sleep disorder (insomnia) and excessive dreaming.
Carbidopa and levodopa may have similar effects on thinking and behavior. This abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Ordinarily, patients with a major psychotic disorder should not be treated with carbidopa and levodopa, because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson’s disease and may decrease the effectiveness of carbidopa and levodopa.
Impulse Control/Compulsive Behaviors
Reports of patients taking dopaminergic medications (medications that increase central dopaminergic tone), suggest that patients may experience an intense urge to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or the caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with carbidopa and levodopa. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking carbidopa and levodopa (see Information for Patients).
Melanoma
Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using carbidopa and levodopa for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Information for Patients
The patient should be informed that this combination product is an immediate-release formulation of carbidopa and levodopa that is designed to begin release of ingredients within 30 minutes. It is important that carbidopa and levodopa be taken at regular intervals according to the schedule outlined by the physician. The patient should be cautioned not to change the prescribed dosage regimen and not to add any additional antiparkinson medications, including other carbidopa and levodopa preparations, without first consulting the physician.
Patients should be advised that sometimes a 'wearing-off' effect may occur at the end of the dosing interval. The physician should be notified if such response poses a problem to lifestyle.
Patients should be advised that occasionally, dark color (red, brown, or black) may appear in saliva, urine, or sweat after ingestion of carbidopa and levodopa. Although the color appears to be clinically insignificant, garments may become discolored.
The patient should be advised that a change in diet to foods that are high in protein may delay the absorption of levodopa and may reduce the amount taken up in the circulation. Excessive acidity also delays stomach emptying, thus delaying the absorption of levodopa. Iron salts (such as in multivitamin tablets) may also reduce the amount of levodopa available to the body. The above factors may reduce the clinical effectiveness of the levodopa or carbidopa and levodopa therapy.
Patients should be alerted to the possibility of sudden onset of sleep during daily activities, in some cases without awareness or warning signs, when they are taking dopaminergic agents, including levodopa. Patients should be advised to exercise caution while driving or operating machinery and that if they have experienced somnolence and/or sudden sleep onset, they must refrain from these activities. (See WARNINGS, Falling Asleep During Activities of Daily Living and Somnolence.)
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, and other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease, including carbidopa and levodopa. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges or other urges while being treated with carbidopa and levodopa. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges, or other intense urges while taking carbidopa and levodopa. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking carbidopa and levodopa (See PRECAUTIONS, Impulse Control/Compulsive Behaviors).
Laboratory Tests
Abnormalities in laboratory tests may include elevations of liver function tests such as alkaline phosphatase, SGOT (AST), SGPT (ALT), lactic dehydrogenase (LDH), and bilirubin. Abnormalities in blood urea nitrogen (BUN) and positive Coombs test have also been reported. Commonly, levels of blood urea nitrogen, creatinine, and uric acid are lower during administration of this combination product than with levodopa.
Carbidopa and levodopa may cause a false-positive reaction for urinary ketone bodies when a test tape is used for determination of ketonuria. This reaction will not be altered by boiling the urine specimen. False-negative tests may result with the use of glucose-oxidase methods of testing for glucosuria.
Cases of falsely diagnosed pheochromocytoma in patients on carbidopa and levodopa therapy have been reported very rarely. Caution should be exercised when interpreting the plasma and urine levels of catecholamines and their metabolites in patients on levodopa or carbidopa and levodopa therapy.
Drug Interactions
Caution should be exercised when the following drugs are administered concomitantly with carbidopa and levodopa.
Symptomatic postural hypotension occurred when carbidopa and levodopa was added to the treatment of a patient receiving antihypertensive drugs. Therefore, when therapy with carbidopa and levodopa is started, dosage adjustment of the antihypertensive drug may be required.
For patients receiving MAO inhibitors (Type A or B), see CONTRAINDICATIONS. Concomitant therapy with selegiline and carbidopa and levodopa may be associated with severe orthostatic hypotension not attributable to carbidopa and levodopa alone (see CONTRAINDICATIONS).
There have been rare reports of adverse reactions, including hypertension and dyskinesia, resulting from the concomitant use of tricyclic antidepressants and carbidopa and levodopa.
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone) and isoniazid may reduce the therapeutic effects of levodopa. In addition, the beneficial effects of levodopa in Parkinson's disease have been reported to be reversed by phenytoin and papaverine. Patients taking these drugs with carbidopa and levodopa should be carefully observed for loss of therapeutic response.
Use of carbidopa and levodopa with dopamine-depleting agents (e.g., reserpine and tetrabenazine) or other drugs known to deplete monoamine stores is not recommended.
Carbidopa and levodopa and iron salts or multivitamins containing iron salts should be coadministered with caution. Iron salts can form chelates with levodopa and carbidopa and consequently reduce the bioavailability of carbidopa and levodopa.
Although metoclopramide may increase the bioavailability of levodopa by increasing gastric emptying, metoclopramide may also adversely affect disease control by its dopamine receptor antagonistic properties.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year bioassay of carbidopa and levodopa, no evidence of carcinogenicity was found in rats receiving doses of approximately two times the maximum daily human dose of carbidopa and four times the maximum daily human dose of levodopa.
In reproduction studies with carbidopa and levodopa, no effects on fertility were found in rats receiving doses of approximately two times the maximum daily human dose of carbidopa and four times the maximum daily human dose of levodopa.
Pregnancy
No teratogenic effects were observed in a study in mice receiving up to 20 times the maximum recommended human dose of carbidopa and levodopa. There was a decrease in the number of live pups delivered by rats receiving approximately two times the maximum recommended human dose of carbidopa and approximately five times the maximum recommended human dose of levodopa during organogenesis. Carbidopa and levodopa caused both visceral and skeletal malformations in rabbits at all doses and ratios of carbidopa/levodopa tested, which ranged from 10 times/5 times the maximum recommended human dose of carbidopa/levodopa to 20 times/10 times the maximum recommended human dose of carbidopa/levodopa.
There are no adequate or well-controlled studies in pregnant women. It has been reported from individual cases that levodopa crosses the human placental barrier, enters the fetus, and is metabolized. Carbidopa concentrations in fetal tissue appeared to be minimal. Use of carbidopa and levodopa in women of childbearing potential requires that the anticipated benefits of the drug be weighed against possible hazards to mother and child.
Nursing Mothers
Levodopa has been detected in human milk. Caution should be exercised when carbidopa and levodopa is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Use of the drug in patients below the age of 18 is not recommended.
Geriatric Use
In the clinical efficacy trials for carbidopa and levodopa, almost half of the patients were older than 65, but few were older than 75. No overall meaningful differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals to adverse drug reactions such as hallucinations cannot be ruled out. There is no specific dosing recommendation based upon clinical pharmacology data as carbidopa and levodopa is titrated as tolerated for clinical effect.
Close