Label: PROPYLTHIOURACIL tablet
- NDC Code(s): 0228-2348-10
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 31, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
WARNING
Severe liver injury and acute liver failure, in some cases fatal, have been reported in patients treated with propylthiouracil. These reports of hepatic reactions include cases requiring liver transplantation in adult and pediatric patients. Propylthiouracil should be reserved for patients who cannot tolerate methimazole and in whom radioactive iodine therapy or surgery are not appropriate treatments for the management of hyperthyroidism. Propylthiouracil may be the treatment of choice when an antithyroid drug is indicated during or just prior to the first trimester of pregnancy (see WARNINGS and PRECAUTIONS).
Close -
DESCRIPTION
Propylthiouracil, USP is one of the thiocarbamide compounds. It is a white, powdery, crystalline substance that has a bitter taste and is very slightly soluble in water. Propylthiouracil is an ...
-
CLINICAL PHARMACOLOGY
Propylthiouracil inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and triiodothyronine that are ...
-
INDICATIONS AND USAGE
Propylthiouracil tablets are indicated: in patients with Graves’ disease with hyperthyroidism or toxic multinodular goiter who are intolerant of methimazole and for whom surgery or radioactive ...
-
CONTRAINDICATIONS
Propylthiouracil is contraindicated in patients who have demonstrated hypersensitivity to the drug or any of the other product components.
-
WARNINGS
Liver Toxicity - Liver injury resulting in liver failure, liver transplantation, or death, has been reported with propylthiouracil therapy in adult and pediatric patients. No cases of liver ...
-
PRECAUTIONS
General - Patients should be instructed to report any symptoms of hepatic dysfunction (anorexia, pruritus, jaundice, light colored stools, dark urine, right upper quadrant pain, etc.) ...
-
ADVERSE REACTIONS
The following adverse reactions have been reported with the use of propylthiouracil. Because these events generally come from voluntary reporting from a population of uncertain size, it is not ...
-
OVERDOSAGE
Signs and Symptoms - Nausea, vomiting, epigastric distress, headache, fever, arthralgia, pruritus, edema, and pancytopenia. Agranulocytosis is the most serious effect. Rarely, exfoliative ...
-
DOSAGE AND ADMINISTRATION
Propylthiouracil is administered orally. The total daily dosage is usually given in 3 equal doses at approximately 8-hour intervals. Adults - The initial dose is 300 mg daily. In patients with ...
-
HOW SUPPLIED
Propylthiouracil tablets, USP are available as follows: 50 mg — Each white, round, tablet imprinted with on one side and 348 and partial bisect on the other side contains 50 mg of ...
-
REFERENCE
International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. 1974; 7; 67-76. Dispense with Medication Guide available at ...
-
Medication Guide
Dispense with Medication Guide available at: www.tevausa.com/medguides - Propylthiouracil (proe" pil thye" oh ure' a sil) Tablets - Rx only - Read this Medication Guide before you start taking ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 0228-2348-10 - Propylthiouracil Tablets, USP - 50 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information

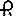 on one side and 348 and partial bisect on the other side contains 50 mg of propylthiouracil, USP. Tablets are supplied in bottles of 100 (NDC 0228-2348-10).
on one side and 348 and partial bisect on the other side contains 50 mg of propylthiouracil, USP. Tablets are supplied in bottles of 100 (NDC 0228-2348-10).