Label: CLONIDINE HYDROCHLORIDE tablet
- NDC Code(s): 0228-2127-10, 0228-2127-50, 0228-2128-10, 0228-2128-50, view more
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
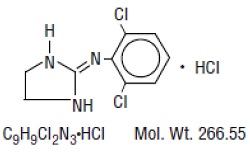
Clonidine hydrochloride, USP is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg, and 0.3 mg. The 0.1 mg ...
-
CLINICAL PHARMACOLOGY
Clonidine stimulates alpha-adrenoreceptors in the brain stem. This action results in reduced sympathetic outflow from the central nervous system and in decreases in peripheral resistance, renal ...
-
INDICATIONS AND USAGE
Clonidine hydrochloride tablets are indicated in the treatment of hypertension. Clonidine hydrochloride tablets may be employed alone or concomitantly with other antihypertensive agents.
-
CONTRAINDICATIONS
Clonidine hydrochloride tablets should not be used in patients with known hypersensitivity to clonidine (see PRECAUTIONS).
-
WARNINGS
Withdrawal: Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such as ...
-
PRECAUTIONS
General: In patients who have developed localized contact sensitization to transdermal clonidine, continuation of transdermal clonidine or substitution of oral clonidine hydrochloride therapy may ...
-
ADVERSE REACTIONS
Most adverse effects are mild and tend to diminish with continued therapy. The most frequent (which appear to be dose-related) are dry mouth, occurring in about 40 of 100 patients; drowsiness ...
-
OVERDOSAGE
Hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis ...
-
DOSAGE AND ADMINISTRATION
Adults: The dose of clonidine hydrochloride tablets must be adjusted according to the patient’s individual blood pressure response. The following is a general guide to its ...
-
HOW SUPPLIED
Clonidine hydrochloride tablets, USP are supplied as follows: 0.1 mg - Each orange, round tablet imprinted with and 127 on one side and bisect on the other side contains 0.1 mg of clonidine ...
-
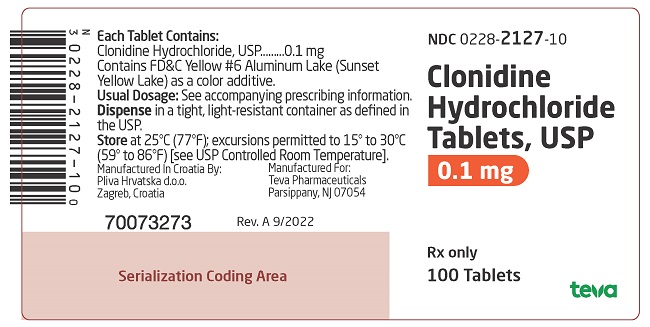
PACKAGE LABEL. PRINCIPAL DISPLAY PANELNDC 0228-2127-10 - Clonidine Hydrochloride Tablets, USP - 0.1 mg - Rx only - 100 Tablets
-
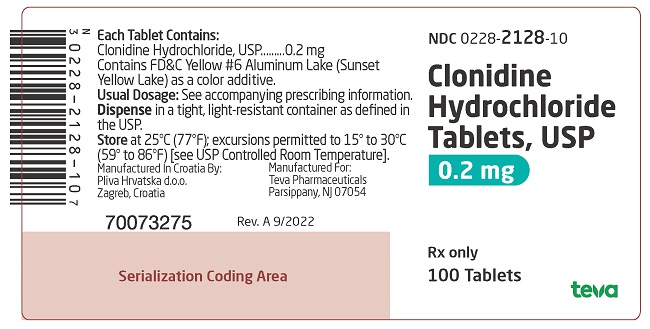
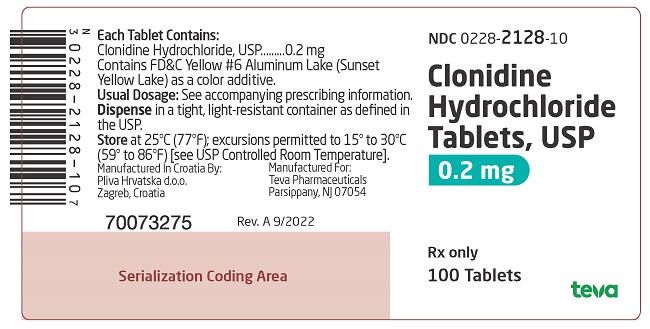
PACKAGE LABEL. PRINCIPAL DISPLAY PANELNDC 0228-2128-10 - Clonidine Hydrochloride Tablets, USP - 0.2 mg - Rx only - 100 Tablets
-
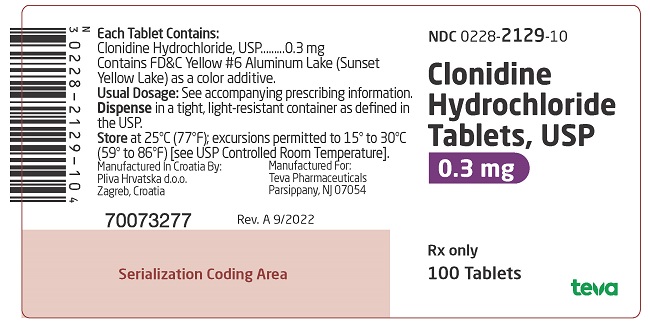
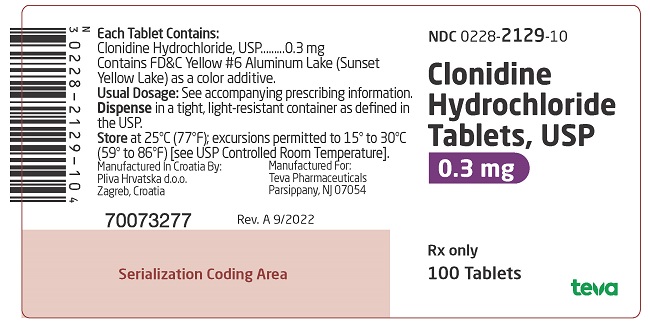
PACKAGE LABEL. PRINCIPAL DISPLAY PANELNDC 0228-2129-10 - Clonidine Hydrochloride Tablets, USP - 0.3 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information

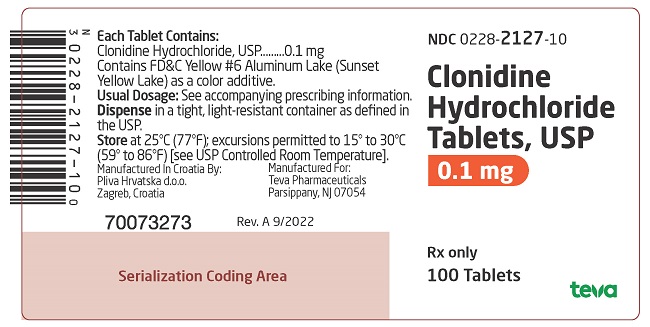
 and 127 on one side and bisect on the other side contains 0.1 mg of clonidine hydrochloride, USP and is supplied in bottles of 100 (NDC 0228-2127-10) and 500 (NDC 0228-2127-50).
and 127 on one side and bisect on the other side contains 0.1 mg of clonidine hydrochloride, USP and is supplied in bottles of 100 (NDC 0228-2127-10) and 500 (NDC 0228-2127-50). on one side and 128 and bisect on the other side contains 0.2 mg of clonidine hydrochloride, USP and is supplied in bottles of 100 (NDC 0228-2128-10) and 500 (NDC 0228-2128-50).
on one side and 128 and bisect on the other side contains 0.2 mg of clonidine hydrochloride, USP and is supplied in bottles of 100 (NDC 0228-2128-10) and 500 (NDC 0228-2128-50). on one side and 129 and bisect on the other side contains 0.3 mg of clonidine hydrochloride, USP and is supplied in bottles of 100 (NDC 0228-2129-10).
on one side and 129 and bisect on the other side contains 0.3 mg of clonidine hydrochloride, USP and is supplied in bottles of 100 (NDC 0228-2129-10).