Label: JUBLIA- efinaconazole solution
- NDC Code(s): 0187-5400-02, 0187-5400-04, 0187-5400-08, 0187-5400-10
- Packager: Bausch Health US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use JUBLIA safely and effectively. See full prescribing information for JUBLIA. JUBLIA® (efinaconazole) topical solution - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEJUBLIA (efinaconazole) topical solution, 10% is an azole antifungal indicated for the topical treatment of onychomycosis of the toenail(s) due to Trichophyton rubrum and Trichophyton ...
-
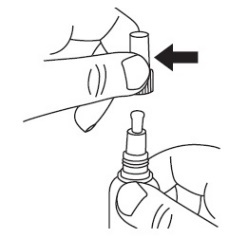
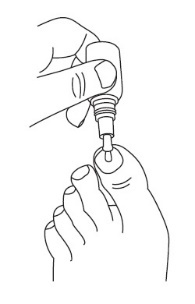
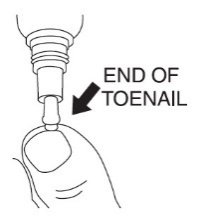
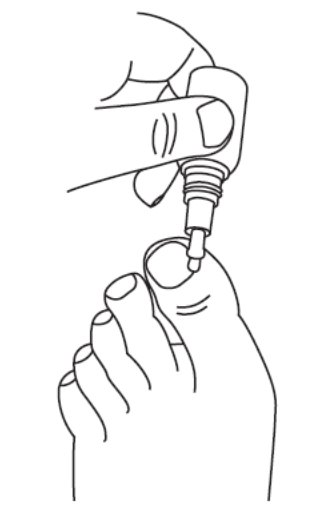
2 DOSAGE AND ADMINISTRATIONApply JUBLIA to affected toenails once daily for 48 weeks, using the integrated flow-through brush applicator. When applying JUBLIA, ensure the toenail, the toenail folds, toenail bed ...
-
3 DOSAGE FORMS AND STRENGTHSJUBLIA (efinaconazole) topical solution, 10% contains 100 mg of efinaconazole in each gram of clear, colorless to pale yellow solution.
-
4 CONTRAINDICATIONSNone.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSIn vitro studies have shown that JUBLIA, at therapeutic concentrations, neither inhibits nor induces cytochrome P450 (CYP450) enzymes.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available human data for the use of JUBLIA during pregnancy to inform any drug associated risks of major birth defects, miscarriage, or adverse ...
-
11 DESCRIPTIONJUBLIA (efinaconazole) topical solution, 10% is a clear, colorless to pale yellow solution for topical use. Each gram of JUBLIA contains 100 mg of efinaconazole. Efinaconazole is an azole ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - JUBLIA topical solution is an azole antifungal [see Clinical Pharmacology (12.4)]. 12.2 Pharmacodynamics - The pharmacodynamics of JUBLIA is unknown. 12.3 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A 2-year dermal carcinogenicity study in mice was conducted with daily topical administration of 3%, 10% and 30% efinaconazole ...
-
14 CLINICAL STUDIESThe safety and efficacy of once daily use of JUBLIA for the treatment of onychomycosis of the toenail were assessed in two 52-week prospective, multicenter, randomized, double-blind clinical ...
-
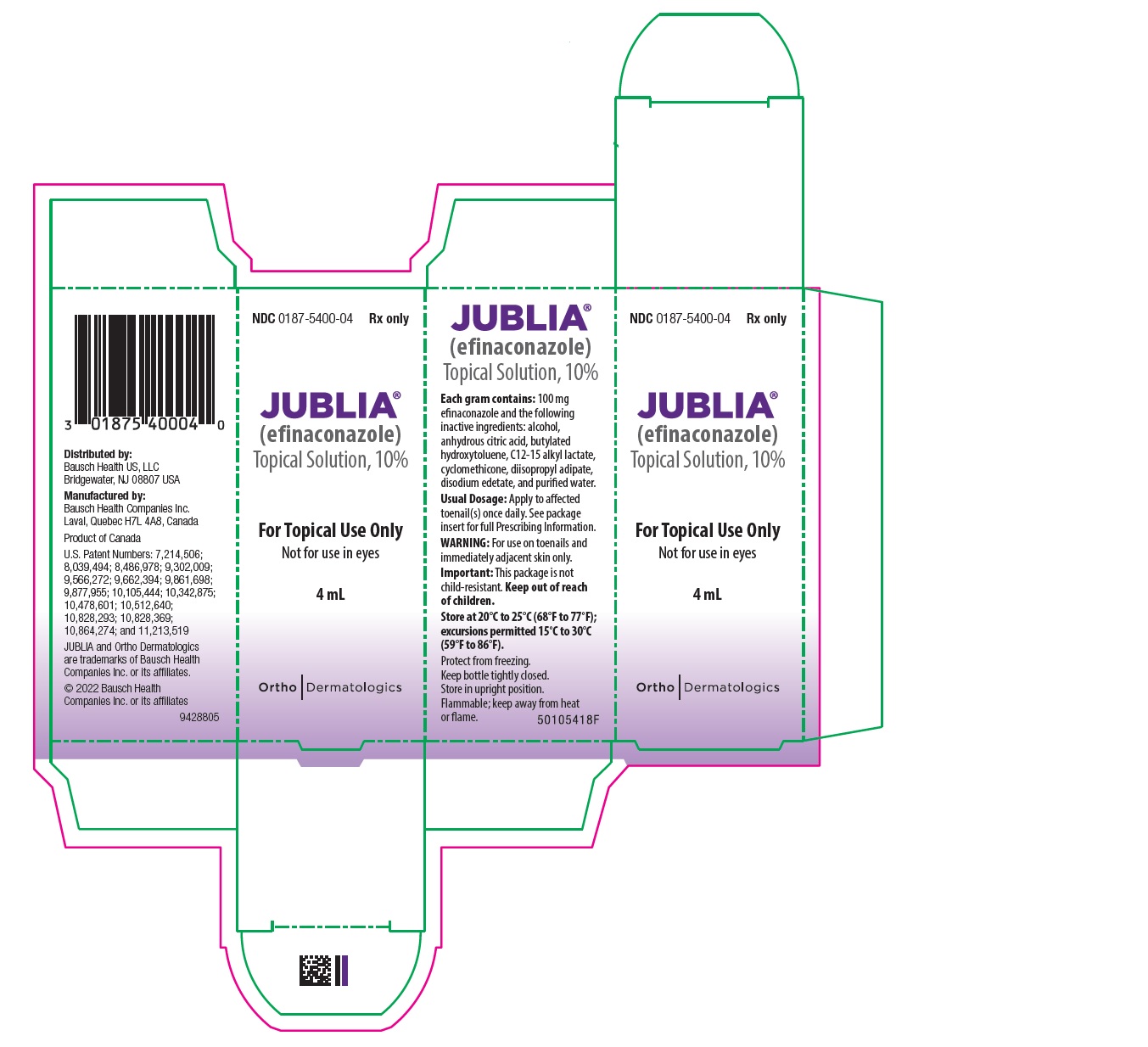
16 HOW SUPPLIED/STORAGE AND HANDLINGJUBLIA (efinaconazole) topical solution, 10% is a clear, colorless to pale yellow solution supplied in a white plastic bottle with an integrated flow-through brush applicator as follows: • 4 mL ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). • JUBLIA is for external use only and is not for oral, ophthalmic, or intravaginal ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Bausch Health US, LLC - Bridgewater, NJ 08807 USA - Manufactured by: Bausch Health Companies Inc. Laval, Quebec H7L 4A8, Canada - U.S. Patent Numbers: 7,214,506; 8,039,494 ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - JUBLIA® (joo-blee-uh) (efinaconazole) topical solution, 10% Important information: JUBLIA is for use on toenails and surrounding skin only. Do not use JUBLIA in your ...
-
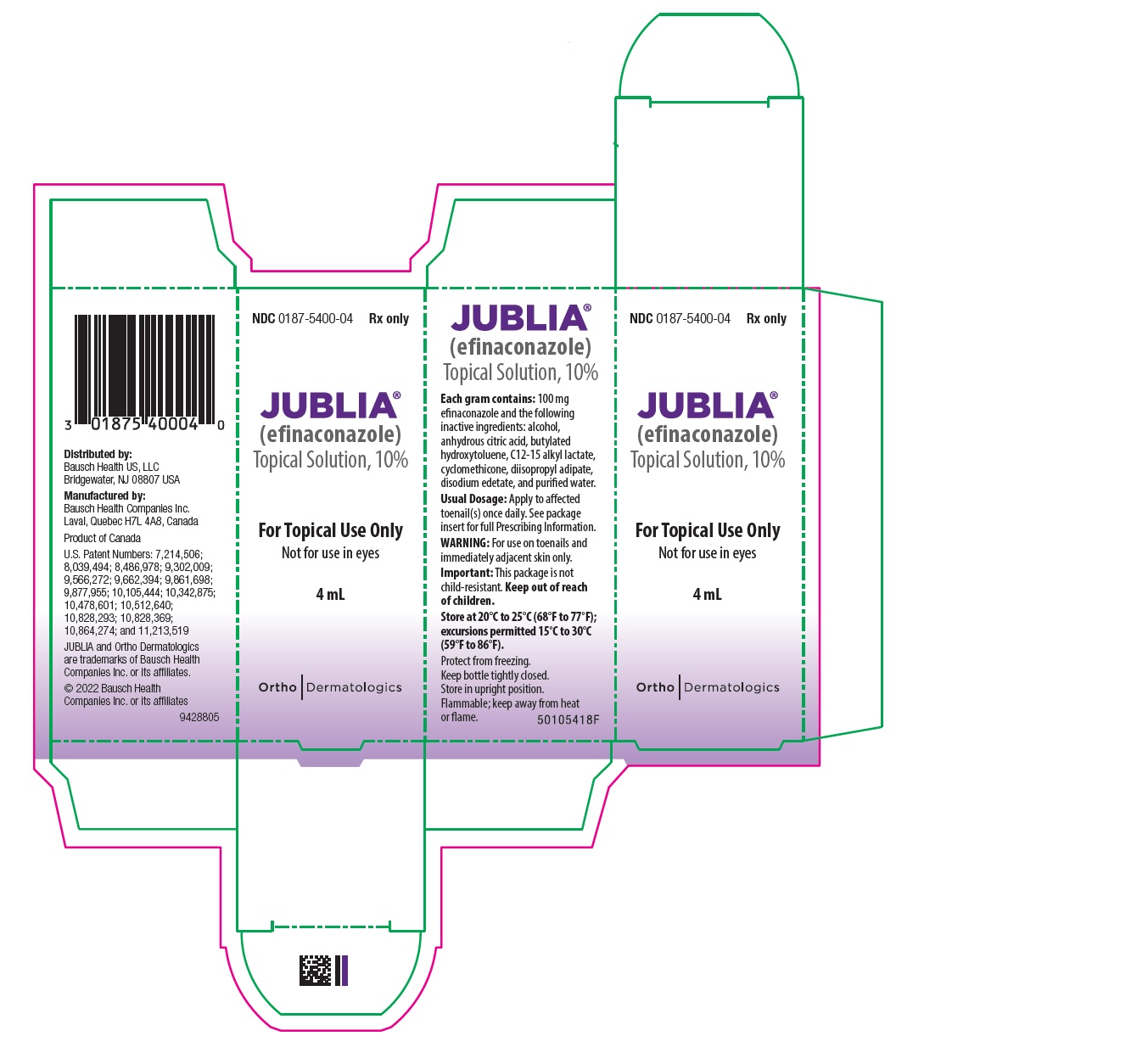
PRINCIPAL DISPLAY PANEL------------------------------------------------------- REPRESENTATIVE PACKAGING - NDC 0187-5400-04 - Rx only - JUBLIA® (efinaconazole) Topical Solution, 10% For Topical Use Only - Not for use ...
-
INGREDIENTS AND APPEARANCEProduct Information