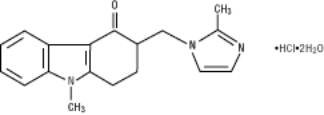
The clinical efficacy of ondansetron hydrochloride, the active ingredient of Ondansetron Injection, USP, was assessed in clinical trials as described below.
14.1 Chemotherapy-Induced Nausea ...
The clinical efficacy of ondansetron hydrochloride, the active ingredient of Ondansetron Injection, USP, was assessed in clinical trials as described below.
14.1 Chemotherapy-Induced Nausea and Vomiting
Adults
In a double-blind trial of three different dosing regimens of ondansetron injection, 0.015 mg/kg, 0.15 mg/kg, and 0.30 mg/kg, each given three times during the course of cancer chemotherapy, the 0.15-mg/kg dosing regimen was more effective than the 0.015-mg/kg dosing regimen. The 0.30-mg/kg dosing regimen was not shown to be more effective than the 0.15-mg/kg dosing regimen.
Cisplatin-Based Chemotherapy
In a double-blind trial in 28 patients, ondansetron injection (three 0.15-mg/kg doses) was significantly more effective than placebo in preventing nausea and vomiting induced by cisplatin-based chemotherapy. Therapeutic response was as shown in Table 7.
Table 7. Therapeutic Response in Prevention of Chemotherapy-induced Nausea and Vomiting in Single-Day Cisplatin Therapy a in Adults
a Chemotherapy was high dose (100 and 120 mg/m2; ondansetron injection n = 6, placebo n = 5) or moderate dose (50 and 80 mg/m2; ondansetron injection n = 8, placebo n = 9). Other chemotherapeutic agents included fluorouracil, doxorubicin, and cyclophosphamide. There was no difference between treatments in the types of chemotherapy that would account for differences in response.
b Efficacy based on “all-patients-treated” analysis.
c Median undefined since at least 50% of the patients were rescued or had more than five emetic episodes.
d Visual analog scale assessment of nausea: 0 = no nausea, 100 = nausea as bad as it can be.
e Visual analog scale assessment of satisfaction: 0 = not at all satisfied, 100 = totally satisfied.
Ondansetron injection (0.15-mg/kg x 3 doses) was compared with metoclopramide (2 mg/kg x 6 doses) in a single-blind trial in 307 patients receiving cisplatin ≥ 100 mg/m2 with or without other chemotherapeutic agents. Patients received the first dose of ondansetron or metoclopramide 30 minutes before cisplatin. Two additional ondansetron doses were administered 4 and 8 hours later, or five additional metoclopramide doses were administered 2, 4, 7, 10, and 13 hours later. Cisplatin was administered over a period of 3 hours or less. Episodes of vomiting and retching were tabulated over the period of 24 hours after cisplatin. The results of this trial are summarized in Table 8.
Table 8. Therapeutic Response in Prevention of Vomiting Induced by Cisplatin (≥ 100 mg/m2) Single-Day Therapy a in Adults
a In addition to cisplatin, 68% of patients received other chemotherapeutic agents, including cyclophosphamide, etoposide, and fluorouracil. There was no difference between treatments in the types of chemotherapy that would account for differences in response.
b Visual analog scale assessment: 0 = not at all satisfied, 100 = totally satisfied.
Cyclophosphamide-Based Chemotherapy
In a double-blind, placebo-controlled trial of ondansetron injection (three 0.15-mg/kg doses) in 20 patients receiving cyclophosphamide (500 to 600 mg/m2) chemotherapy, ondansetron injection was significantly more effective than placebo in preventing nausea and vomiting. The results are summarized in Table 9.
Table 9. Therapeutic Response in Prevention of Chemotherapy-induced Nausea and Vomiting in Single-Day Cyclophosphamide Therapy a in Adults
a Chemotherapy consisted of cyclophosphamide in all patients, plus other agents, including fluorouracil, doxorubicin, methotrexate, and vincristine. There was no difference between treatments in the type of chemotherapy that would account for differences in response.
b Efficacy based on “all-patients-treated” analysis.
c Median undefined since at least 50% of patients did not have any emetic episodes.
d Visual analog scale assessment of nausea: 0 = no nausea, 100 = nausea as bad as it can be.
e Visual analog scale assessment of satisfaction: 0 = not at all satisfied, 100 = totally satisfied.
Re-treatment
In uncontrolled trials, 127 patients receiving cisplatin (median dose, 100 mg/m2) and ondansetron who had two or fewer emetic episodes were re-treated with ondansetron and chemotherapy, mainly cisplatin, for a total of 269 re-treatment courses (median: 2; range, 1 to 10). No emetic episodes occurred in 160 (59%), and two or fewer emetic episodes occurred in 217 (81%) re-treatment courses.
Pediatrics
Four open-label, noncomparative (one US, three foreign) trials have been performed with 209 pediatric cancer patients aged 4 to 18 years given a variety of cisplatin or noncisplatin regimens. In the three foreign trials, the initial ondansetron injection dose ranged from 0.04 to 0.87 mg/kg for a total dose of 2.16 to 12 mg. This was followed by the oral administration of ondansetron ranging from 4 to 24 mg daily for 3 days. In the US trial, ondansetron was administered intravenously (only) in three doses of 0.15 mg/kg each for a total daily dose of 7.2 to 39 mg. In these trials, 58% of the 196 evaluable patients had a complete response (no emetic episodes) on Day 1. Thus, prevention of vomiting in these pediatric patients was essentially the same as for patients older than 18 years.
An open-label, multicenter, noncomparative trial has been performed in 75 pediatric cancer patients aged 6 to 48 months receiving at least one moderately or highly emetogenic chemotherapeutic agent. Fifty-seven percent (57%) were females; 67% were white, 18% were American Hispanic, and 15% were black patients. Ondansetron was administered intravenously over 15 minutes in three doses of 0.15 mg/kg. The first dose was administered 30 minutes before the start of chemotherapy; the second and third doses were administered 4 and 8 hours after the first dose, respectively. Eighteen patients (25%) received routine prophylactic dexamethasone (i.e., not given as rescue). Of the 75 evaluable patients, 56% had a complete response (no emetic episodes) on Day 1. Thus, prevention of vomiting in these pediatric patients was comparable to the prevention of vomiting in patients aged 4 years and older.
14.2 Prevention of Postoperative Nausea and/or Vomiting
Adults
Adult surgical patients who received ondansetron immediately before the induction of general balanced anesthesia (barbiturate: thiopental, methohexital, or thiamylal; opioid: alfentanil or fentanyl; nitrous oxide; neuromuscular blockade: succinylcholine/curare and/or vecuronium or atracurium; and supplemental isoflurane) were evaluated in two double-blind US trials involving 554 patients.
Ondansetron injection (4 mg) intravenous given over 2 to 5 minutes was significantly more effective than placebo. The results of these trials are summarized in Table 10.
Table 10. Therapeutic Response in Prevention of Postoperative Nausea and Vomiting in Adult Patients
The populations in Table 10 consisted mainly of females undergoing laparoscopic procedures.
In a placebo-controlled trial conducted in 468 males undergoing outpatient procedures, a single 4-mg intravenous ondansetron dose prevented postoperative vomiting over a 24-hour period in 79% of males receiving drug compared with 63% of males receiving placebo (P < 0.001).
Two other placebo-controlled trials were conducted in 2,792 patients undergoing major abdominal or gynecological surgeries to evaluate a single 4-mg or 8-mg intravenous ondansetron dose for prevention of postoperative nausea and vomiting over a 24-hour period. At the 4-mg dosage, 59% of patients receiving ondansetron versus 45% receiving placebo in the first trial (P < 0.001) and 41% of patients receiving ondansetron versus 30% receiving placebo in the second trial (P = 0.001) experienced no emetic episodes. No additional benefit was observed in patients who received intravenous ondansetron 8 mg compared with patients who received intravenous ondansetron 4 mg.
Pediatrics
Three double-blind, placebo-controlled trials have been performed (one US, two foreign) in 1,049 male and female patients (aged 2 to 12 years) undergoing general anesthesia with nitrous oxide. The surgical procedures included tonsillectomy with or without adenoidectomy, strabismus surgery, herniorrhaphy, and orchidopexy. Patients were randomized to either single intravenous doses of ondansetron (0.1 mg/kg for pediatric patients weighing 40 kg or less, 4 mg for pediatric patients weighing more than 40 kg) or placebo. Study drug was administered over at least 30 seconds, immediately prior to or following anesthesia induction. Ondansetron was significantly more effective than placebo in preventing nausea and vomiting. The results of these trials are summarized in Table 11.
Table 11. Therapeutic Response in Prevention of Postoperative Nausea and Vomiting in Pediatric Patients Aged 2 to 12 Years
a Failure was one or more emetic episodes, rescued, or withdrawn.
b Nausea measured as none, mild, or severe.
A double-blind, multicenter, placebo-controlled trial was conducted in 670 pediatric patients aged 1 month to 24 months who were undergoing routine surgery under general anesthesia. Seventy-five percent (75%) were males; 64% were white, 15% were black, 13% were American Hispanic, 2% were Asian, and 6% were “other race” patients. A single 0.1‑mg/kg intravenous dose of ondansetron administered within 5 minutes following induction of anesthesia was statistically significantly more effective than placebo in preventing vomiting. In the placebo group, 28% of patients experienced vomiting compared with 11% of subjects who received ondansetron (P ≤ 0.01). Overall, 32 (10%) of placebo patients and 18 (5%) of patients who received ondansetron received antiemetic rescue medication(s) or prematurely withdrew from the trial.
14.3 Prevention of Further Postoperative Nausea and Vomiting
Adults
Adult surgical patients receiving general balanced anesthesia (barbiturate: thiopental, methohexital, or thiamylal; opioid: alfentanil or fentanyl; nitrous oxide; neuromuscular blockade: succinylcholine/curare and/or vecuronium or atracurium; and supplemental isoflurane) who received no prophylactic antiemetics and who experienced nausea and/or vomiting within 2 hours postoperatively were evaluated in two double-blind US trials involving 441 patients. Patients who experienced an episode of postoperative nausea and/or vomiting were given ondansetron injection (4 mg) intravenously over 2 to 5 minutes, and this was significantly more effective than placebo. The results of these trials are summarized in Table 12.
Table 12. Therapeutic Response in Prevention of Further Postoperative Nausea and Vomiting in Adult Patients
a After administration of study drug.
b Nausea measured on a scale of 0-10 with 0 = no nausea, 10 = nausea as bad as it can be.
The populations in Table 12 consisted mainly of women undergoing laparoscopic procedures.
Repeat Dosing in Adults
In patients who do not achieve adequate control of postoperative nausea and vomiting following a single, prophylactic, preinduction, intravenous dose of ondansetron 4 mg, administration of a second intravenous dose of ondansetron 4 mg postoperatively does not provide additional control of nausea and vomiting.
Pediatrics
One double-blind, placebo-controlled, US trial was performed in 351 male and female outpatients (aged 2 to 12 years) who received general anesthesia with nitrous oxide and no prophylactic antiemetics. Surgical procedures were unrestricted. Patients who experienced two or more emetic episodes within 2 hours following discontinuation of nitrous oxide were randomized to either single intravenous doses of ondansetron (0.1 mg/kg for pediatric patients weighing 40 kg or less, 4 mg for pediatric patients weighing more than 40 kg) or placebo administered over at least 30 seconds. Ondansetron was significantly more effective than placebo in preventing further episodes of nausea and vomiting. The results of the trials are summarized in Table 13.
Table 13. Therapeutic Response in Prevention of Further Postoperative Nausea and Vomiting in Pediatric Patients Aged 2 to 12 Years
a Failure was one or more emetic episodes, rescued, or withdrawn.
Close





![NDC 0143-9891-01 ONDANSETRON INJECTION, USP 4 mg/2 mL (2 mg/mL) STERILE FOR IV OR IM USE Rx ONLY 2 mL Single Dose Vial USUAL DOSAGE: See package insert. Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]. Protect from light.](/dailymed/image.cfm?name=ondansetron-injection-2.jpg&setid=bb4bc810-db26-471b-9ce7-6e2fb3018af0)
![NDC 0143-9890-01 ONDANSETRON INJECTION, USP 40 mg/20 mL (2 mg/mL) STERILE FOR IV OR IM USE Rx ONLY 20 mL Multiple Dose Vial Each 1 mL of aqueous solution in the 20 mL multidose vial contains 2 mg of ondansetron as the hydrochloride dihydrate; 8.3 mg of Sodium Chloride, USP; 0.5 mg of Citric Acid Monohydrate, USP and 0.25 mg Trisodium Citrate Dihydrate, USP as buffers; and 1.2 mg of Methylparaben, NF and 0.15 mg of propylparaben as preservatives in Water for Injection, USP. USUAL DOSAGE: See package insert. Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]. Protect from light.](/dailymed/image.cfm?name=ondansetron-injection-3.jpg&setid=bb4bc810-db26-471b-9ce7-6e2fb3018af0)
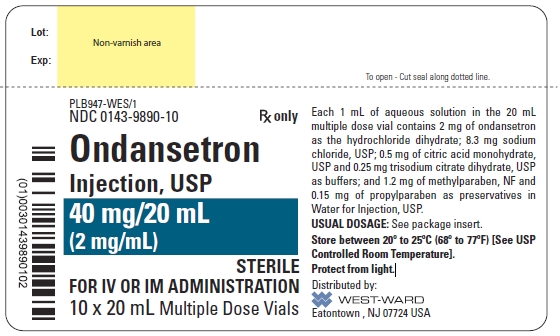
![NDC 0143-9890-10 Rx only Ondansetron Injection, USP 40 mg/20 mL (2 mg/mL) STERILE FOR IV OR IM ADMINISTRATION 10 x 20 mL Multiple Dose Vials Each 1 mL of aqueous solution in the 20 mL multiple dose vial contains 2 mg of ondansetron as the hydrochloride dihydrate; 8.3 mg sodium chloride, USP; 0.5 mg of citric acid monohydrate, USP and 0.25 mg trisodium citrate dihydrate, USP as buffers; and 1.2 mg of methylparaben, NF and 0.15 mg of propylparaben as preservatives in Water for Injection, USP. USUAL DOSAGE: See package insert. Store between 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]. Protect from light.](/dailymed/image.cfm?name=ondansetron-injection-4.jpg&setid=bb4bc810-db26-471b-9ce7-6e2fb3018af0)