Label: AMOXICILLIN AND CLAVULANATE POTASSIUM suspension
- NDC Code(s): 0143-9853-16, 0143-9853-24, 0143-9853-75
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMOXICILLIN AND CLAVULANATE POTASSIUM FOR ORAL SUSPENSION USP, 600 mg/42.9 mg per 5 mL safely and effectively. See full prescribing ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL is indicated for the treatment of pediatric patients with - Recurrent or persistent acute otitis media due to S ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions - To minimize the potential for gastrointestinal intolerance, amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL should ...
-
3 DOSAGE FORMS AND STRENGTHS
Amoxicillin and Clavulanate Potassium for Oral Suspension USP, 600 mg/42.9 mg per 5 mL: orange-flavored powder for oral suspension (each 5 mL of reconstituted suspension contains 600 mg ...
-
4 CONTRAINDICATIONS
4.1 Serious Hypersensitivity Reactions - Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL is contraindicated in patients with a history of serious ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Allergic Reactions, Including Anaphylaxis - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam ...
-
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling [see Warnings and Precautions (5)]: Anaphylactic reactions [see Warnings and Precautions (5.1)] Severe Cutaneous ...
-
7 DRUG INTERACTIONS
7.1 Probenecid - Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use with amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL may ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - There are no adequate and well-controlled studies of amoxicillin and clavulanate potassium in pregnant women. Because animal reproduction studies are not always predictive of ...
-
10 OVERDOSAGE
Following overdosage, patients have experienced primarily gastrointestinal symptoms including stomach and abdominal pain, vomiting, and diarrhea. Rash, hyperactivity, or drowsiness have also been ...
-
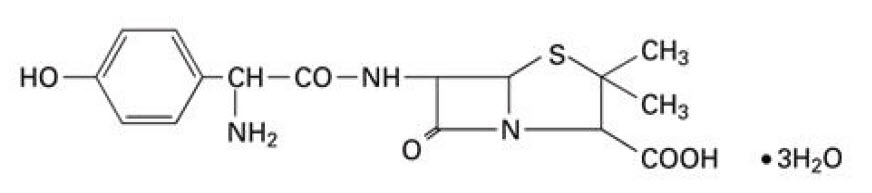
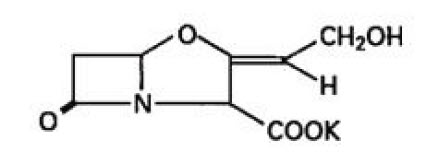
11 DESCRIPTION
Amoxicillin and Clavulanate Potassium for Oral Suspension USP, 600 mg/42.9 mg per 5 mL is an oral antibacterial combination consisting of the semisynthetic antibacterial amoxicillin and the ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL is an antibacterial drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - The ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Amoxicillin and clavulanate potassium (4:1 ...
-
14 CLINICAL STUDIES
Two clinical studies were conducted in pediatric patients with acute otitis media. A noncomparative, open-label study assessed the bacteriologic and clinical efficacy of amoxicillin and ...
-
15 REFERENCES
1. Swanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988;30:66-67.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - Amoxicillin and Clavulanate Potassium for Oral Suspension USP, 600 mg/42.9 mg per 5 mL: Each 5 mL of reconstituted orange-flavored suspension contains 600 mg amoxicillin ...
-
17 PATIENT COUNSELING INFORMATION
Administration Instructions - Inform patients to take amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL every 12 hours with a meal or snack to reduce the ...
-
PRINCIPAL DISPLAY PANELNDC 0143-9853-75 - Amoxicillin and Clavulanate Potassium - for Oral Suspension, USP - 600 mg/42.9 mg* per 5 mL - 75 mL (when reconstituted) Rx only
-
PRINCIPAL DISPLAY PANELNDC 0143-9853-16 - Amoxicillin and Clavulanate Potassium - for Oral Suspension, USP - 600 mg/42.9 mg* per 5 mL - 125 mL (when reconstituted) Rx only
-
PRINCIPAL DISPLAY PANELNDC 0143-9853-24 - Amoxicillin and Clavulanate Potassium - for Oral Suspension, USP - 600 mg/42.9 mg* per 5 mL - 200 mL (when reconstituted) Rx only
-
INGREDIENTS AND APPEARANCEProduct Information