Label: PROGESTERONE injection
- NDC Code(s): 0143-9725-01
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
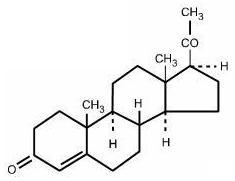
Progesterone injection, USP, a progestin, is a sterile solution of progesterone in a suitable vegetable oil available for intramuscular use. Progesterone occurs as a white or creamy white ...
-
CLINICAL PHARMACOLOGYTransforms proliferative endometrium into secretory endometrium. Inhibits (at the usual dose range) the secretion of pituitary gonadotropins, which in turn prevents follicular maturation and ...
-
INDICATIONS AND USAGE
This drug is indicated in amenorrhea and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as submucous fibroids or uterine cancer.
-
CONTRAINDICATIONS
Current or past history of thrombophlebitis, thromboembolic disorders, or cerebral apoplexy. Liver dysfunction or disease. Known or suspected malignancy of breast or genital ...
-
WARNINGS
The physician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, cerebrovascular disorders, pulmonary embolism, and retinal thrombosis). Should any of these ...
-
PRECAUTIONS
General - The pretreatment physical examination should include special reference to breast and pelvic organs, as well as a Papanicolaou smear. Because progestational drugs may cause some degree ...
-
ADVERSE REACTIONS
Breakthrough bleeding; spotting; change in menstrual flow; amenorrhea; edema; change in weight (increase or decrease); changes in cervical erosion and cervical secretions; cholestatic jaundice ...
-
DOSAGE AND ADMINISTRATION
Progesterone Injection, USP is administered by intramuscular injection. It differs from other commonly used steroids in that it is irritating at the place of injection. Amenorrhea: Five to 10 mg ...
-
HOW SUPPLIED
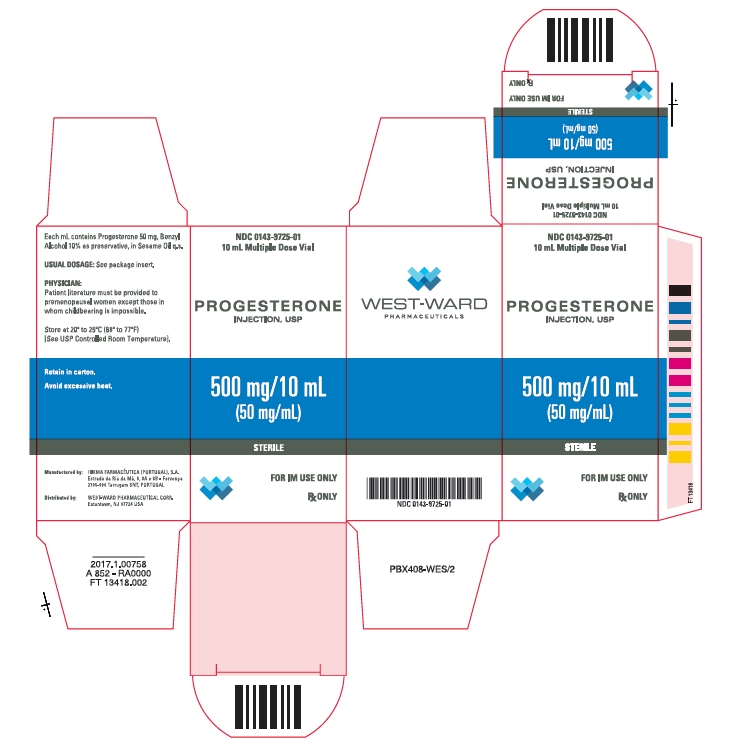
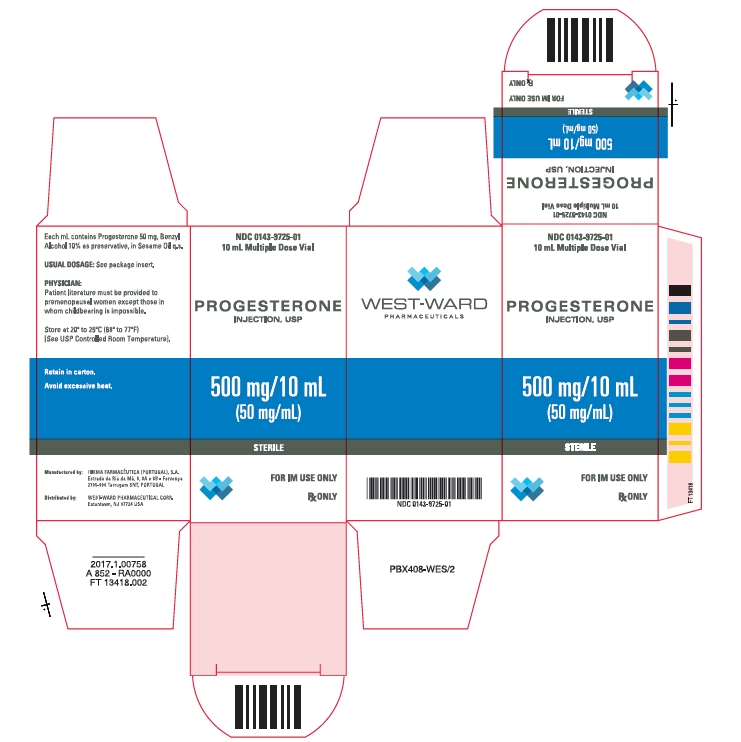
Progesterone Injection, USP, 50 mg/mL is available in 10 mL multiple dose vials, individually boxed. (NDC 0143-9725-01) Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room ...
-
PATIENT INFORMATION
PROGESTERONE INJECTION, USP - IN SESAME OIL – FOR INTRAMUSCULAR USE ONLY - Rx Only - FOR THE TREATMENT OF AMENORRHEA (ABSENCE OF MENSES IN WOMEN WHO HAVE PREVIOUSLY HAD A MENSTRUAL PERIOD) OR ...
-
PRINCIPAL DISPLAY PANELNDC 0143-9725-01 - PROGESTERONE - INJECTION, USP - 500 mg/10 mL - (50 mg/mL) STERILE - FOR IM USE ONLY - Rx ONLY - NDC 0143-9725-01 - 10 mL Multiple Dose Vial - PROGESTERONE - INJECTION, USP - 500 ...
-
INGREDIENTS AND APPEARANCEProduct Information