Label: CHLORHEXIDINE GLUCONATE rinse
- NDC Code(s): 0121-0893-00, 0121-0893-04, 0121-0893-15, 0121-0893-16, view more
- Packager: PAI Holdings, LLC dba PAI Pharma
- This is a repackaged label.
- Source NDC Code(s): 66689-106
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
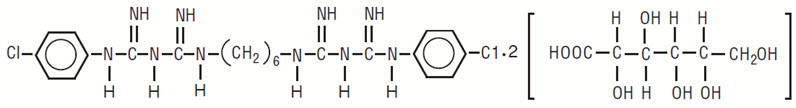
DESCRIPTIONChlorhexidine gluconate oral rinse USP, 0.12% is an oral rinse containing 0.12% chlorhexidine gluconate (1,1’-hexamethylene bis [5-(p-chlorophenyl) biguanide]di-D-gluconate) in a base containing ...
-
CLINICAL PHARMACOLOGYChlorhexidine gluconate oral rinse, 0.12% provides antimicrobial activity during oral rinsing. The clinical significance of chlorhexidine gluconate oral rinse, 0.12% antimicrobial activities is ...
-
INDICATIONChlorhexidine gluconate oral rinse, 0.12% is indicated for use between dental visits as part of a professional program for the treatment of gingivitis as characterized by redness and swelling of ...
-
CONTRAINDICATIONSChlorhexidine gluconate oral rinse, 0.12% should not be used by persons who are known to be hypersensitive to chlorhexidine gluconate or other formula ingredients.
-
WARNINGSThe effect of chlorhexidine gluconate oral rinse, 0.12% on periodontitis has not been determined. An increase in supragingival calculus was noted in clinical testing in chlorhexidine gluconate ...
-
PRECAUTIONSGeneral - 1. For patients having coexisting gingivitis and periodontitis, the presence or absence of gingival inflammation following treatment with chlorhexidine gluconate oral rinse, 0.12 ...
-
ADVERSE REACTIONSThe most common side effects associated with chlorhexidine gluconate oral rinses are: 1) an increase in staining of teeth and other oral surfaces; 2) an increase in calculus formation; and 3) an ...
-
OVERDOSAGEIngestion of 1 or 2 ounces of chlorhexidine gluconate oral rinse, 0.12% by a small child (~10 kg body weight) might result in gastric distress, including nausea, or signs of alcohol intoxication ...
-
DOSAGE AND ADMINISTRATIONChlorhexidine gluconate oral rinse, 0.12% therapy should be initiated directly following a dental prophylaxis. Patients using chlorhexidine gluconate oral rinse, 0.12% should be reevaluated and ...
-
HOW SUPPLIEDChlorhexidine gluconate oral rinse USP, 0.12% is a clear, light blue, peppermint flavored liquid and is supplied as: NDC 0121-0893-04: 4 fl oz (118 mL) NDC 0121-0893-16: 16 fl oz (473 mL) NDC ...
-
SPL UNCLASSIFIED SECTIONMANUFACTURED BY - Pharmaceutical Associates, Inc. Greenville, SC 29605 - www.paipharma.com - R03/24
-
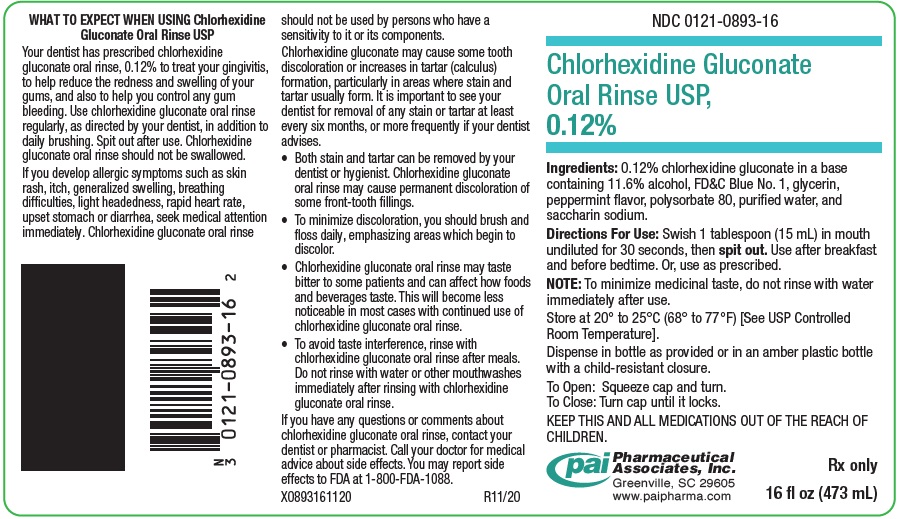
SPL UNCLASSIFIED SECTIONWhat To Expect When Using Chlorhexidine Gluconate Oral Rinse, USP 0.12% Your dentist has prescribed chlorhexidine gluconate oral rinse, 0.12% to treat your gingivitis, to help reduce the redness ...
-
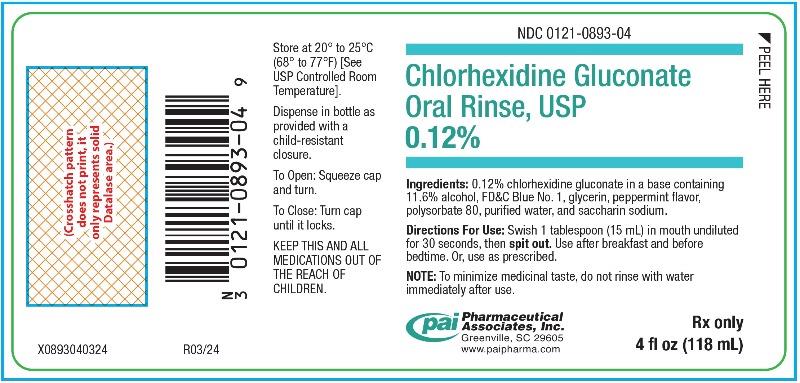
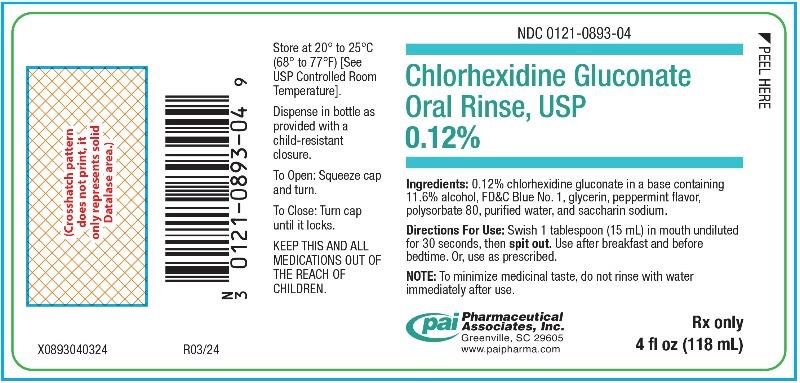
PRINCIPAL DISPLAY PANEL - 4 oz (118 mL) Bottle Label
NDC 0121-0893-04 - Chlorhexidine Gluconate - Oral Rinse, USP - 0.12% Ingredients: 0.12% chlorhexidine gluconate in a base containing - 11.6% alcohol, FD&C Blue No. 1, glycerin, peppermint ...
-
PRINCIPAL DISPLAY PANEL - 4 oz (118 mL) CartonNDC 0121-0893-04 - Chlorhexidine - Gluconate - Oral Rinse, USP - 0.12% Ingredients: 0.12% chlorhexidine gluconate - in a base containing 11.6% alcohol, FD&C - Blue No. 1, glycerin, peppermint ...
-
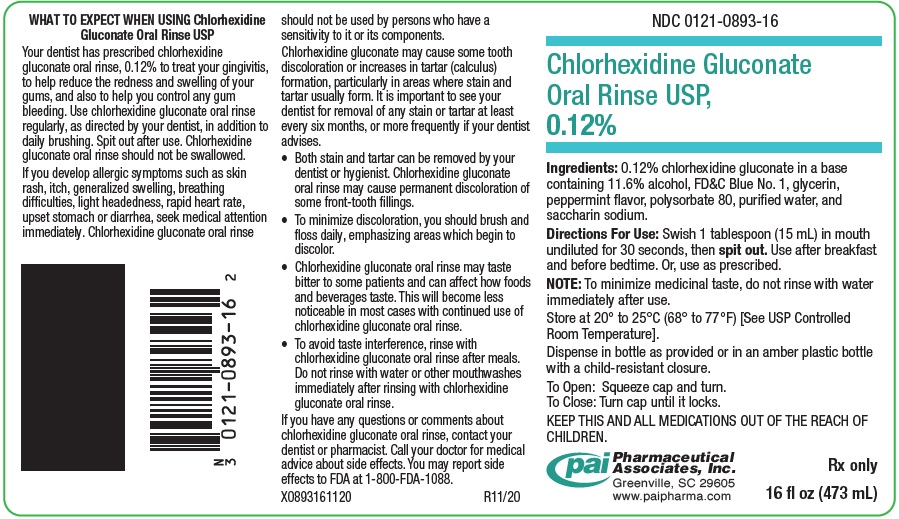
PRINCIPAL DISPLAY PANEL - 16 oz (473 mL) Bottle LabelNDC 0121-0893-16 - Chlorhexidine Gluconate - Oral Rinse USP, 0.12% Ingredients: 0.12% chlorhexidine gluconate in a base - containing 11.6% alcohol, FD&C Blue No. 1, glycerin, peppermint ...
-
PRINCIPAL DISPLAY PANEL - 15 mL Cup LabelDelivers 15 mL - NDC 0121-0893-15 - Chlorhexidine - Gluconate - Oral Rinse USP, 0.12% Package Not Child-Resistant - Rx ONLY - PHARMACEUTICAL ASSOCIATES, INC. GREENVILLE, SC 29605 - SEE ...
-
INGREDIENTS AND APPEARANCEProduct Information