Label: TRICITRATES- potassium citrate, sodium citrate, and citric acid monohydrate solution
- NDC Code(s): 0121-0677-16
- Packager: PAI Holdings, LLC dba PAI Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx ONLY
-
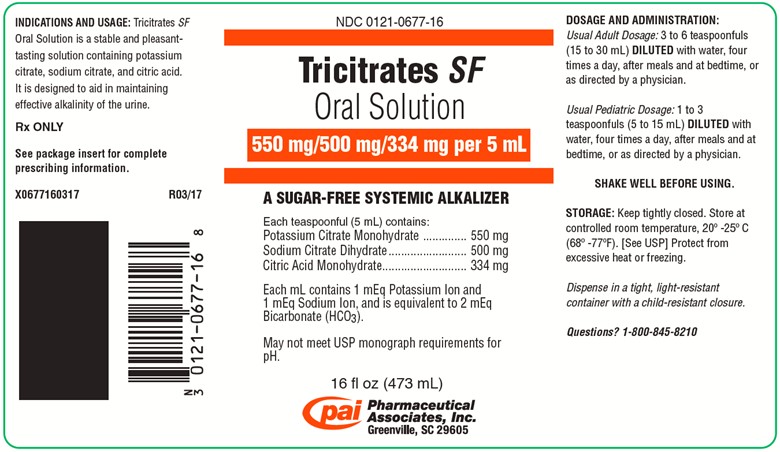
DESCRIPTIONTricitrates - SF Oral Solution is a stable and pleasant-tasting oral systemic alkalizer containing potassium citrate, sodium citrate, and citric acid in a sugar-free ...
-
ACTIONSPotassium citrate and sodium citrate are absorbed and metabolized to potassium bicarbonate and sodium bicarbonate, thus acting as systemic alkalizers. The effects are essentially those of ...
-
INDICATIONS AND ADVANTAGESTricitrates - SF Oral Solution is an effective alkalinizing agent useful in those conditions where long-term maintenance of an alkaline urine is desirable, such as in ...
-
CONTRAINDICATIONSSevere renal impairment with oliguria or azotemia, untreated Addison's disease, or severe myocardial damage. In certain situations, when patients are on a sodium-restricted diet, the use of ...
-
PRECAUTIONS AND WARNINGSShould be used with caution by patients with low urinary output or reduced glomerular filtration rates unless under the supervision of a physician. Aluminum-based antacids should be avoided in ...
-
ADVERSE REACTIONSTricitrates - SF Oral Solution is generally well tolerated without any unpleasant side effects when given in recommended doses to patients with normal renal function and ...
-
DOSAGE AND ADMINISTRATIONTricitrates - SF Oral Solution should be taken diluted in water, followed by additional water, if desired. Palatability is enhanced if chilled before taking ...
-
OVERDOSAGEOverdosage with sodium salts may cause diarrhea, nausea and vomiting, hypernoia, and convulsions. Overdosage with potassium salts may cause hyperkalemia and alkalosis, especially in the presence ...
-
HOW SUPPLIEDTricitrates - SF Oral Solution (orange colored, raspberry flavored) is supplied in the following oral dosage form: NDC 0121-0677-16: 16 fl oz (473 ...
-
SPL UNCLASSIFIED SECTIONManufactured By - Pharmaceutical Associates, Inc. Greenville, SC 29605 - R08/22
-
PRINCIPAL DISPLAY PANEL - 473 mL Bottle LabelNDC 0121-0677-16 - Tricitrates SF - Oral Solution - 550 mg/500 mg/334 mg per 5 mL - A SUGAR-FREE SYSTEMIC ALKALIZER - Each teaspoonful (5 mL) contains ...
-
INGREDIENTS AND APPEARANCEProduct Information