Label: FLUPHENAZINE HYDROCHLORIDE elixir
- NDC Code(s): 0121-0654-02, 0121-0654-16
- Packager: PAI Holdings, LLC dba PAI Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx ONLY
-
BOXED WARNING
(What is this?)
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Fluphenazine Hydrochloride Elixir USP is not approved for the treatment of patients with dementia-related psychosis ( see WARNINGS).
Close -
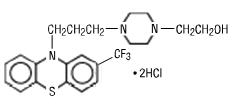
DESCRIPTIONFluphenazine hydrochloride is a trifluoromethyl phenothiazine derivative intended for the management of schizophrenia. Fluphenazine hydrochloride is described chemically as ...
-
CLINICAL PHARMACOLOGYFluphenazine hydrochloride has activity at all levels of the central nervous system as well as on multiple organ systems. The mechanism whereby its therapeutic action is exerted is unknown.
-
INDICATIONS AND USAGEFluphenazine hydrochloride elixir is indicated in the management of manifestations of psychotic disorders. Fluphenazine hydrochloride has not been shown effective in the management of behavioral ...
-
CONTRAINDICATIONSPhenothiazines are contraindicated in patients with suspected or established subcortical brain damage, in patients receiving large doses of hypnotics, and in comatose or severely depressed states ...
-
WARNINGSIncreased Mortality in Elderly Patients with Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death ...
-
PRECAUTIONSGeneral - Because of the possibility of cross-sensitivity, fluphenazine hydrochloride should be used cautiously in patients who have developed cholestatic jaundice, dermatoses or other allergic ...
-
ADVERSE REACTIONSCentral Nervous System - The side effects most frequently reported with phenothiazine compounds are extrapyramidal symptoms including pseudoparkinsonism, dystonia, dyskinesia, akathisia ...
-
DOSAGE AND ADMINISTRATIONDepending on the severity and duration of symptoms, total daily dosage for adult psychotic patients may range initially from 2.5 to 10.0 mg and should be divided and given at six- to ...
-
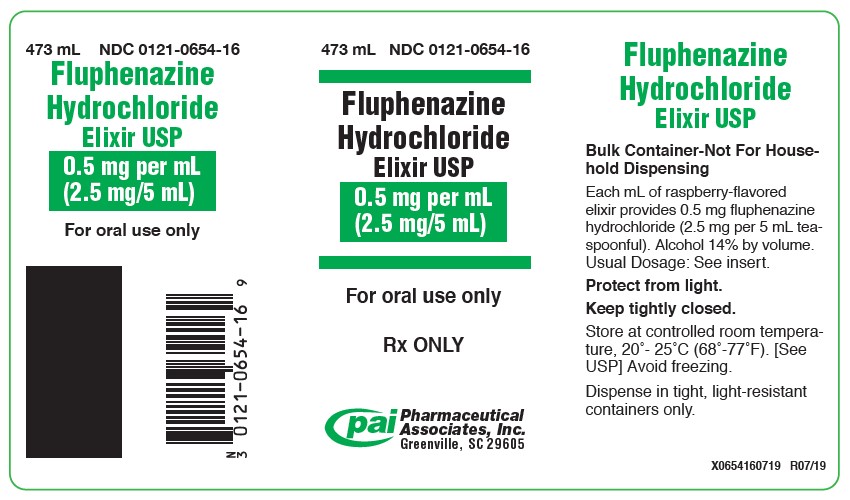
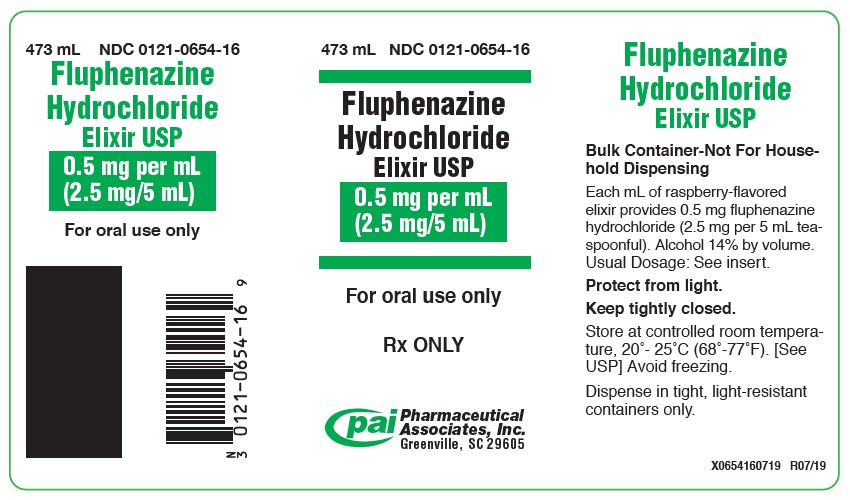
HOW SUPPLIEDFluphenazine Hydrochloride Elixir USP 0.5 mg/mL (2.5 mg per 5 mL teaspoonful), available as an orange-colored, raspberry flavored elixir and is supplied in the following sizes: 60 mL bottle with ...
-
SPL UNCLASSIFIED SECTIONDistributed by: PAI Pharma - Greenville, SC 29605 - www.paipharma.com - R11/24
-
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Label60 mL NDC 0121-0654-02 - Fluphenazine - Hydrochloride - Elixir USP - 0.5 mg per mL - (2.5 mg/5 mL) Rx ONLY - PAI Pharma
-
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label473 mL NDC 0121-0654-16 - Fluphenazine - Hydrochloride - Elixir USP - 0.5 mg per mL - (2.5 mg/5 mL) For oral use only - Rx ONLY - PAI Pharma
-
INGREDIENTS AND APPEARANCEProduct Information