Label: LEVALBUTEROL- levalbuterol hydrochloride solution
- NDC Code(s): 0115-9930-76, 0115-9930-78, 0115-9931-76, 0115-9931-78, view more
- Packager: Amneal Pharmaceuticals of New York LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 29, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVALBUTEROL INHALATION SOLUTION, USP safely and effectively. See full prescribing information for LEVALBUTEROL INHALATION SOLUTION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Levalbuterol Inhalation Solution, USP is indicated for the treatment or prevention of bronchospasm in adults, adolescents, and children 6 years of age and older with reversible obstructive airway ...
-
2 DOSAGE AND ADMINISTRATION
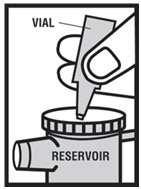
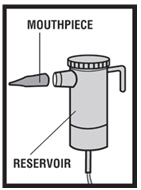
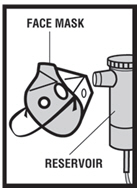
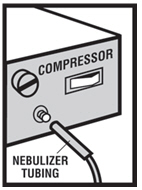
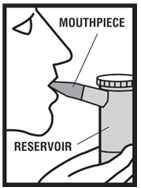
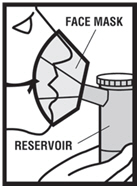
Levalbuterol Inhalation Solution, USP is for oral inhalation only. Administer by nebulization using a standard jet nebulizer (with a face mask or mouthpiece) connected to an air compressor. Do not ...
-
3 DOSAGE FORMS AND STRENGTHS
Inhalation Solution 3 mL unit-dose, vials in three dosage strengths of levalbuterol; 0.31 mg, 0.63 mg, 1.25 mg.
-
4 CONTRAINDICATIONS
Levalbuterol Inhalation Solution, USP is contraindicated in patients with a history of hypersensitivity to levalbuterol or racemic albuterol. Reactions have included urticaria, angioedema, rash ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Paradoxical Bronchospasm - Levalbuterol Inhalation Solution, USP can produce paradoxical bronchospasm, which may be life-threatening. If paradoxical bronchospasm occurs, Levalbuterol ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling: Paradoxical bronchospasm [see - Warnings and Precautions (5.1) ...
-
7 DRUG INTERACTIONS
7.1 Short-Acting Bronchodilators - Avoid concomitant use of other short-acting sympathomimetic bronchodilators or epinephrine in patients being treated with Levalbuterol Inhalation Solution, USP ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Teratogenic Effects: Pregnancy Category C. There are no adequate and well-controlled studies of Levalbuterol Inhalation Solution, USP in pregnant women. Because animal ...
-
10 OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic receptor stimulation and/or occurrence or exaggeration of any of the symptoms listed under - Adverse ...
-
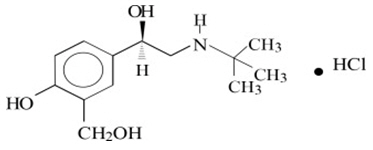
11 DESCRIPTION
Levalbuterol Inhalation Solution, USP is a sterile, clear, colorless, preservative-free solution of the hydrochloride salt of levalbuterol, the (R)-enantiomer of the drug substance racemic ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Activation of beta2-adrenergic receptors on airway smooth muscle leads to the activation of adenylate cyclase and to an increase in the intracellular concentration of ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Although there have been no carcinogenesis studies with levalbuterol HCl, racemic albuterol sulfate has been evaluated for its ...
-
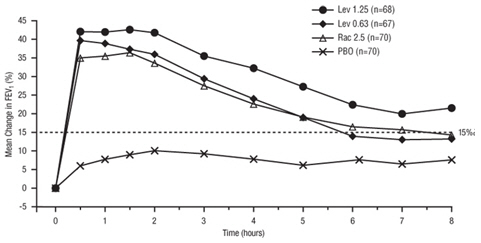
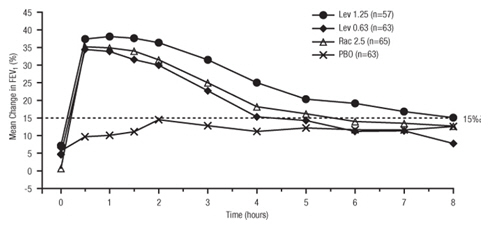
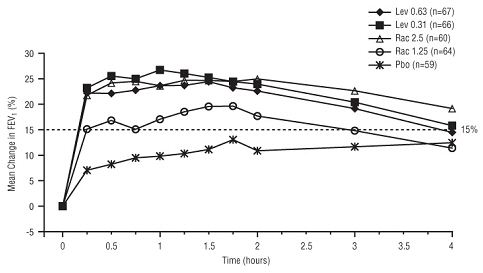
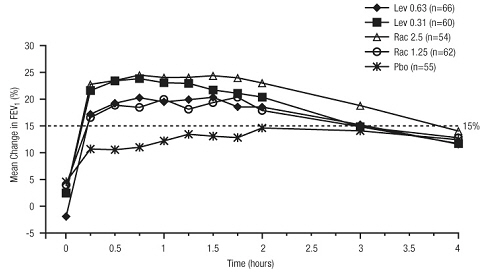
14 CLINICAL STUDIES
Adults and Adolescents ≥ 12 Years Old - The safety and efficacy of Levalbuterol Inhalation Solution, USP were evaluated in a 4-week, multicenter, randomized, double-blind, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Levalbuterol Inhalation Solution, USP is supplied in 3 mL unit-dose, low-density polyethylene (LDPE) vials as a clear, colorless, sterile, preservative-free, aqueous solution, in three different ...
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling - (Patient Information Leaflet and - Instructions for Using Levalbuterol Inhalation Solution, USP). Patients should be ...
-
SPL UNCLASSIFIED SECTIONManufactured By: The Ritedose Corporation - Columbia, SC 29203 USA - Distributed by: Amneal Pharmaceuticals LLC - Bridgewater, NJ 08807 - To report SUSPECTED ADVERSE REACTIONS, contact Amneal ...
-
PATIENT INFORMATION LEAFLET
Levalbuterol Inhalation Solution, USP - (pronounced lev" al bue' ter ol) 0.31 mg, 0.63 mg, 1.25 mg - 3 mL Unit-Dose Vials - For Oral Inhalation Only - Rx only - Levalbuterol Inhalation ...
-
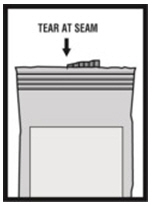
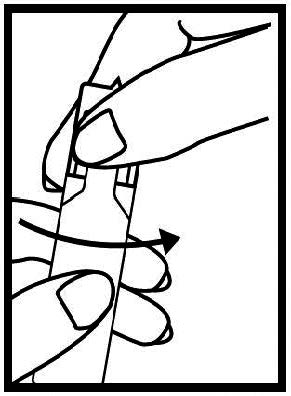
Instructions for Using Levalbuterol Inhalation Solution, USP
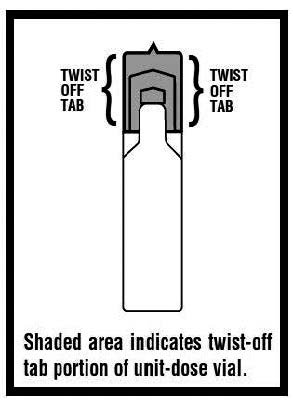
Levalbuterol Inhalation Solution, USP vial (see - Figure A): Figure A - Using your Levalbuterol Inhalation Solution, USP: Read the following Steps before using your Levalbuterol ...
-
PRINCIPAL DISPLAY PANEL - 0.31 mg 24 Vials Carton
NDC 0115-9930-78 - Levalbuterol Inhalation Solution, USP - 0.31 mg (0.0103%) 0.31 mg/3 mL - Rx only
-
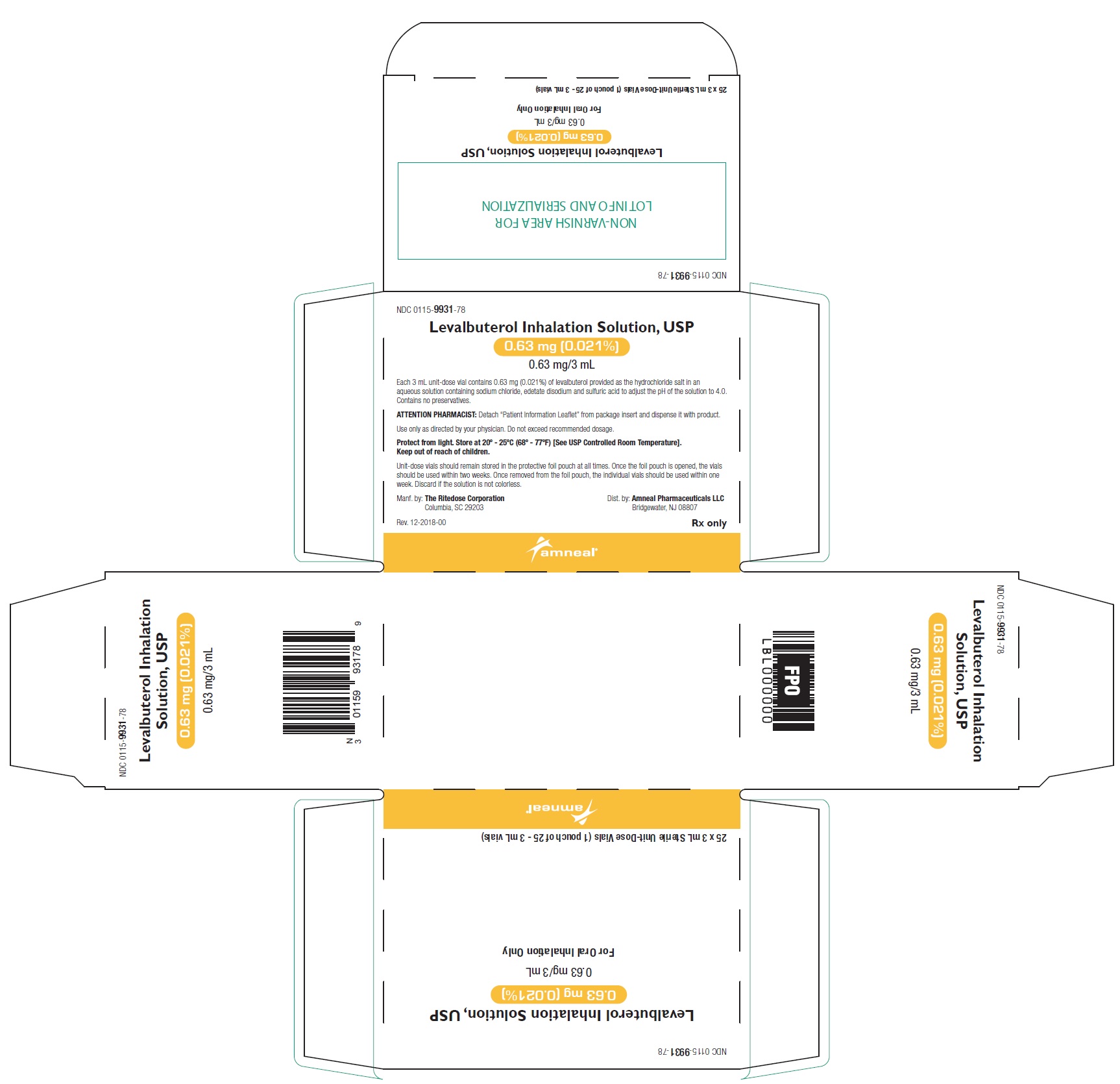
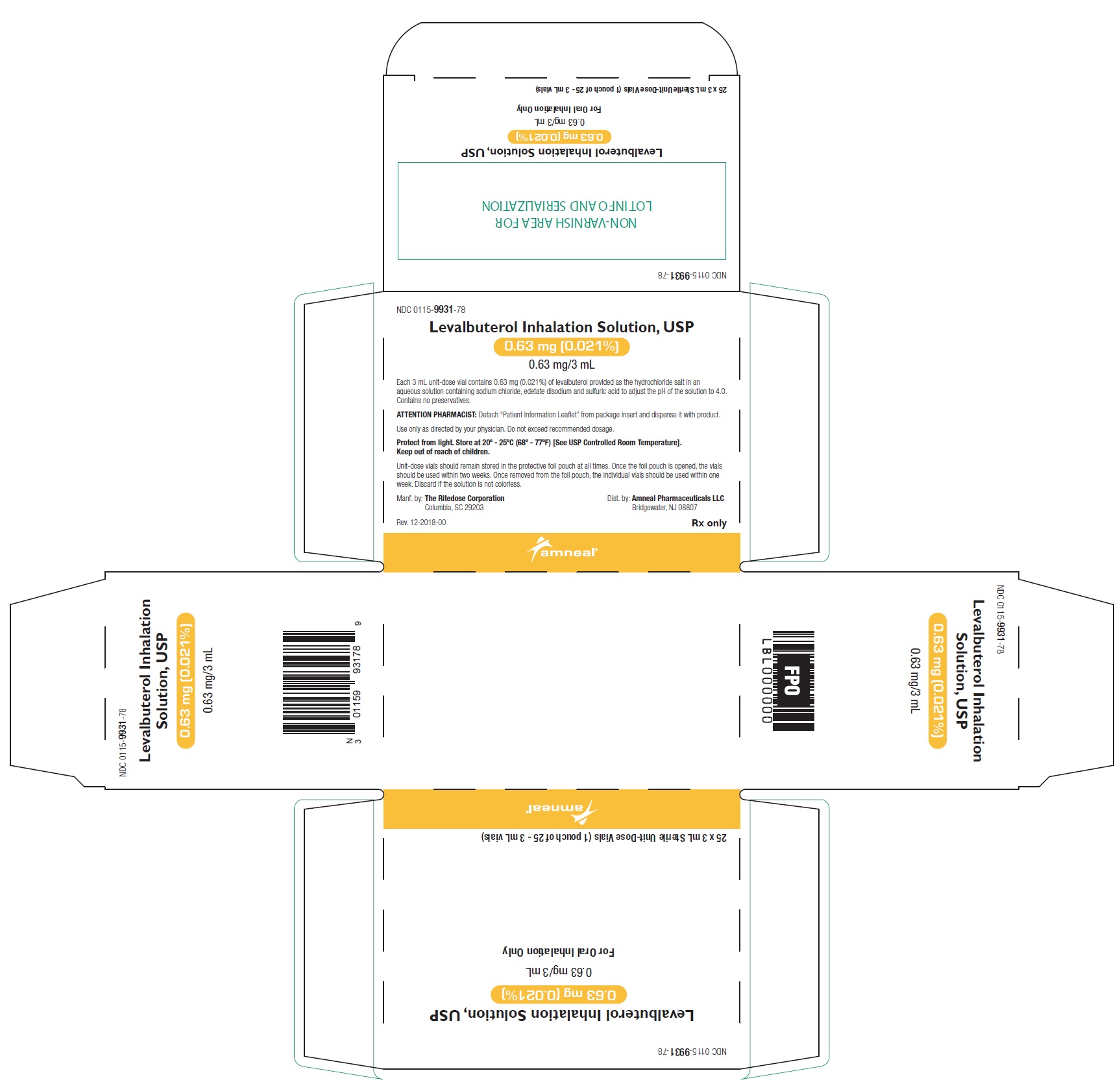
PRINCIPAL DISPLAY PANEL - 0.63 mg 24 Vials Carton
NDC 0115-9931-78 - Levalbuterol Inhalation Solution, USP - 0.63 mg (0.021%) 0.63 mg/3 mL - Rx only
-
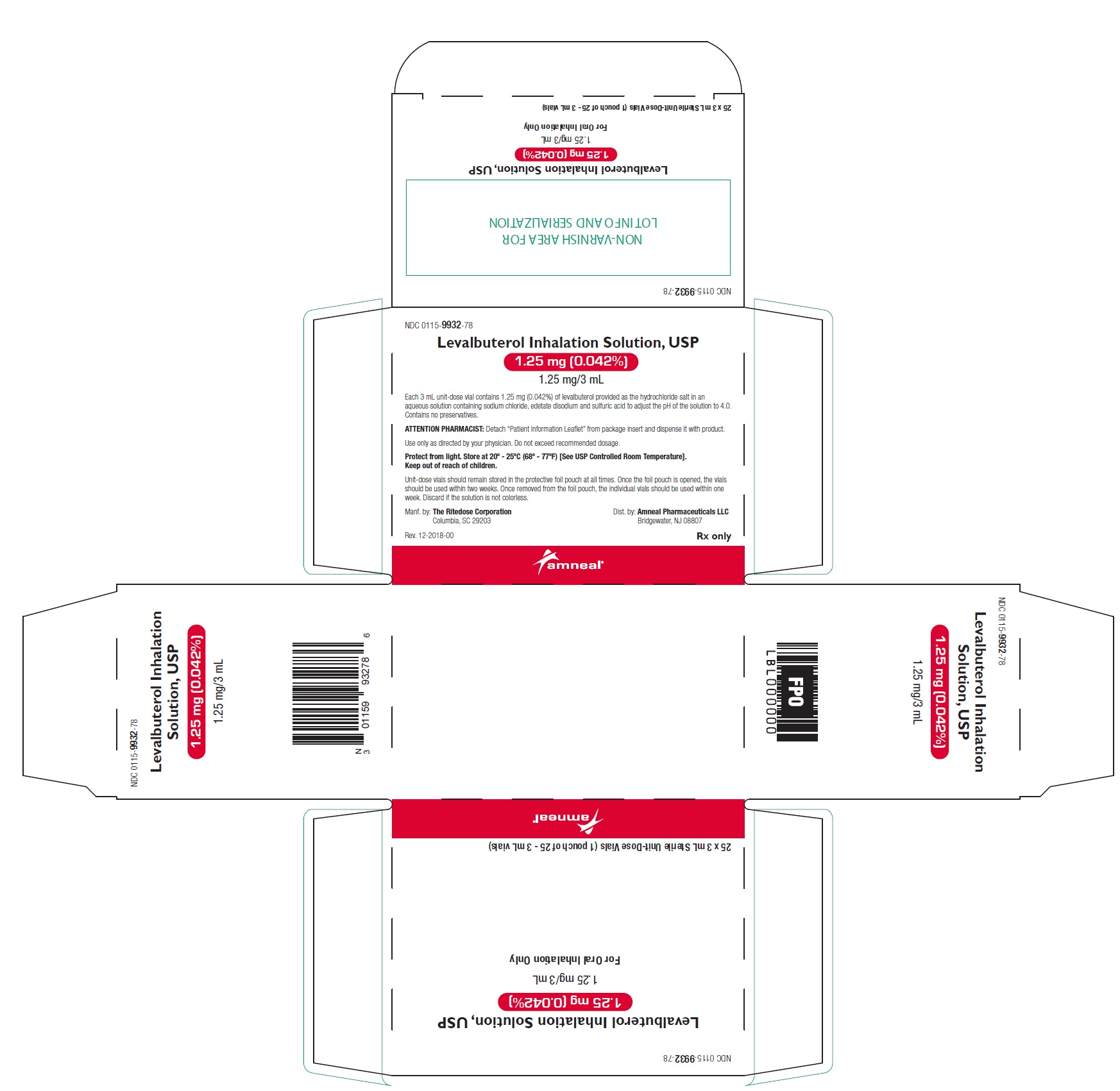
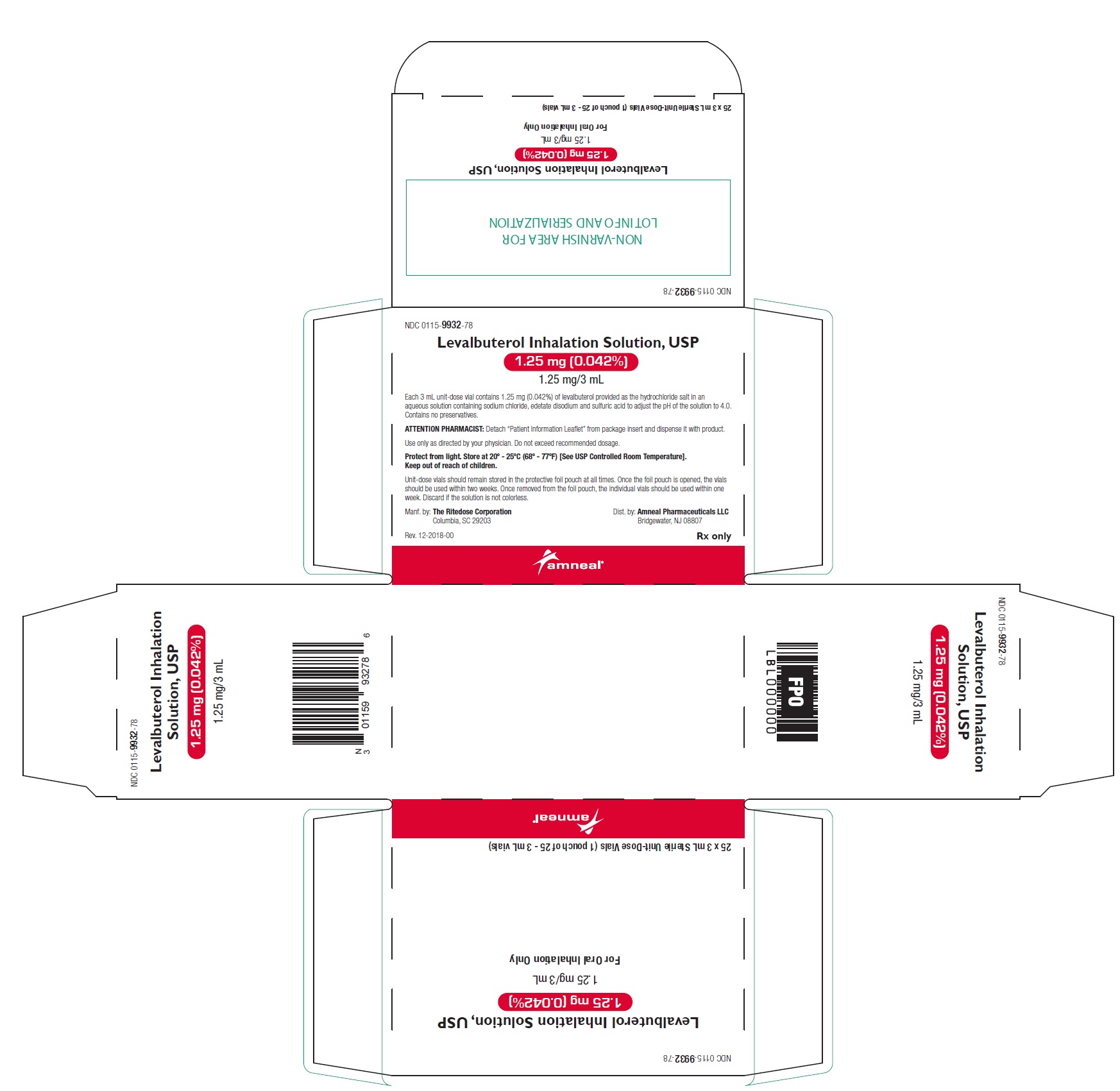
PRINCIPAL DISPLAY PANEL - 1.25 mg 24 Vials Carton
NDC 0115-9932-78 - Levalbuterol Inhalation Solution, USP - 1.25 mg (0.042%) 1.25 mg/3 mL - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information