Label: HYDROXYZINE PAMOATE capsule
- NDC Code(s): 0115-1803-01, 0115-1803-02, 0115-1804-01, 0115-1804-02
- Packager: Amneal Pharmaceuticals of New York LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 21, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
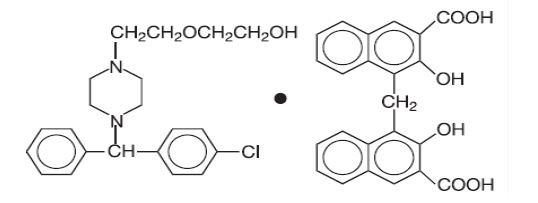
DESCRIPTIONHydroxyzine pamoate, USP is a light yellow, practically odorless powder, practically insoluble in water and methanol and freely soluble in dimethylformamide. It is chemically designated as ...
-
CLINICAL PHARMACOLOGYHydroxyzine pamoate is unrelated chemically to the phenothiazines, reserpine, meprobamate, or the benzodiazepines. Hydroxyzine pamoate is not a cortical depressant, but its action may be due to a ...
-
INDICATIONSFor symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested. Useful in the management of pruritus due to ...
-
CONTRAINDICATIONSHydroxyzine, when administered to the pregnant mouse, rat, and rabbit, induced fetal abnormalities in the rat and mouse at doses substantially above the human therapeutic range. Clinical data in ...
-
WARNINGSNursing Mothers: It is not known whether this drug is excreted in human milk. Since many drugs are so excreted, hydroxyzine should not be given to nursing mothers.
-
PRECAUTIONSTHE POTENTIATING ACTION OF HYDROXYZINE MUST BE CONSIDERED WHEN THE DRUG IS USED IN CONJUNCTION WITH CENTRAL NERVOUS SYSTEM DEPRESSANTS SUCH AS NARCOTICS, NON-NARCOTIC ANALGESICS AND BARBITURATES ...
-
ADVERSE REACTIONSSide effects reported with the administration of hydroxyzine pamoate are usually mild and transitory in nature. Skin and Appendages: Oral hydroxyzine hydrochloride is associated with Acute ...
-
OVERDOSAGEThe most common manifestation of overdosage of hydroxyzine pamoate is hypersedation. Other reported signs and symptoms were convulsions, stupor, nausea and vomiting. As in the management of ...
-
DOSAGEFor symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested: in adults, 50 mg to 100 mg q.i.d.; children ...
-
HOW SUPPLIEDHydroxyzine pamoate capsules, USP (hydroxyzine pamoate equivalent to hydroxyzine hydrochloride) are supplied as follows: 25 mg capsules: Dark green opaque cap/light green opaque body filled with ...
-
BIBLIOGRAPHYAvailable on request. Brands listed are the trademarks of their respective owners. Manufactured by: Patheon Pharmaceuticals Inc. Cincinnati, OH 45237 USA - OR - Manufactured by: Amneal ...
-
PRINCIPAL DISPLAY PANEL - 25 mg
 ...
... -
PRINCIPAL DISPLAY PANEL - 50 mg
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information