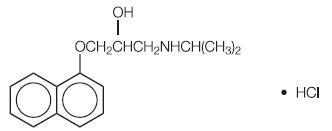
Metabolism and Elimination
Propranolol is extensively metabolized with most metabolites appearing in the urine. Propranolol is metabolized through three primary routes: aromatic hydroxylation (mainly 4-hydroxylation), N-dealkylation followed by further side-chain oxidation, and direct glucuronidation. It has been estimated that the percentage contributions of these routes to total metabolism are 42%, 41% and 17%, respectively, but with considerable variability between individuals. The four major metabolites are propranolol glucuronide, naphthyloxylactic acid and glucuronic acid, and sulfate conjugates of 4-hydroxy propranolol.
In vitro studies have indicated that the aromatic hydroxylation of propranolol is catalyzed mainly by polymorphic CYP2D6. Side-chain oxidation is mediated mainly by CYP1A2 and to some extent by CYP2D6. 4-hydroxy propranolol is a weak inhibitor of CYP2D6.
Propranolol is also a substrate of CYP2C19 and a substrate for the intestinal efflux transporter, p-glycoprotein (p-gp). Studies suggest however that p-gp is not dose-limiting for intestinal absorption of propranolol in the usual therapeutic dose range.
In healthy subjects, no difference was observed between CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs) with respect to oral clearance or elimination half-life. Partial clearance of 4-hydroxy propranolol was significantly higher and of naphthyloxyactic acid significantly lower in EMs than PMs.
The plasma half-life of propranolol is from 3 to 6 hours.
Special Populations
Geriatric
In a study of 12 elderly (62 to 79 years old) and 12 young (25 to 33 years old) healthy subjects, the clearance of S(-)-enantiomer of propranolol was decreased in the elderly. Additionally, the half-life of both the R(+)- and S(-)-propranolol were prolonged in the elderly compared with the young (11 hours vs. 5 hours).
Clearance of propranolol is reduced with aging due to decline in oxidation capacity (ring oxidation and side-chain oxidation). Conjugation capacity remains unchanged. In a study of 32 patients age 30 to 84 years given a single 20-mg dose of propranolol, an inverse correlation was found between age and the partial metabolic clearances to 4-hydroxypropranolol (40HP-ring oxidation) and to naphthoxylactic acid (NLA-side chain oxidation). No correlation was found between age and the partial metabolic clearance to propranolol glucuronide (PPLG-conjugation).
Gender
In a study of 9 healthy women and 12 healthy men, neither the administration of testosterone nor the regular course of the menstrual cycle affected the plasma binding of the propranolol enantiomers. In contrast, there was a significant, although non-enantioselective diminution of the binding of propranolol after treatment with ethinyl estradiol. These findings are inconsistent with another study, in which administration of testosterone cypionate confirmed the stimulatory role of this hormone on propranolol metabolism and concluded that the clearance of propranolol in men is dependent on circulating concentrations of testosterone. In women, none of the metabolic clearances for propranolol showed any significant association with either estradiol or testosterone.
Race
A study conducted in 12 Caucasian and 13 African-American male subjects taking propranolol, showed that at steady state, the clearance of R(+)- and S(-)-propranolol were about 76% and 53% higher in African-Americans than in Caucasians, respectively.
Chinese subjects had a greater proportion (18% to 45% higher) of unbound propranolol in plasma compared to Caucasians, which was associated with a lower plasma concentration of alpha1 acid glycoprotein.
Renal Insufficiency
In a study conducted in 5 patients with chronic renal failure, 6 patients on regular dialysis, and 5 healthy subjects, who received a single oral dose of 40 mg of propranolol, the peak plasma concentrations (Cmax) of propranolol in the chronic renal failure group were 2 to 3-fold higher (161±41 ng/mL) than those observed in the dialysis patients (47±9 ng/mL) and in the healthy subjects (26±1 ng/mL). Propranolol plasma clearance was also reduced in the patients with chronic renal failure.
Studies have reported a delayed absorption rate and a reduced half-life of propranolol in patients with renal failure of varying severity. Despite this shorter plasma half-life, propranolol peak plasma levels were 3 to 4 times higher and total plasma levels of metabolites were up to 3 times higher in these patients than in subjects with normal renal function.
Chronic renal failure has been associated with a decrease in drug metabolism via downregulation of hepatic cytochrome P450 activity resulting in a lower “first-pass” clearance.
Propranolol is not significantly dialyzable.
Hepatic Insufficiency
Propranolol is extensively metabolized by the liver. In a study conducted in 7 patients with cirrhosis and 9 healthy subjects receiving 80-mg oral propranolol every 8 hours for 7 doses, the steady-state unbound propranolol concentration in patients with cirrhosis was increased 3-fold in comparison to controls. In cirrhosis, the half-life increased to 11 hours compared to 4 hours (see PRECAUTIONS).
Drug Interactions
Interactions with Substrates, Inhibitors or Inducers of Cytochrome P-450 Enzymes
Because propranolol’s metabolism involves multiple pathways in the cytochrome P-450 system (CYP2D6, 1A2, 2C19), coadministration with drugs that are metabolized by, or effect the activity (induction or inhibition) of one or more of these pathways may lead to clinically relevant drug interactions (see Drug Interactions under PRECAUTIONS).
Substrates or Inhibitors of CYP2D6
Blood levels and/or toxicity of propranolol may be increased by coadministration with substrates or inhibitors of CYP2D6, such as amiodarone, cimetidine, delavudin, fluoxetine, paroxetine, quinidine, and ritonavir. No interactions were observed with either ranitidine or lansoprazole.
Substrates or Inhibitors of CYP1A2
Blood levels and/or toxicity of propranolol may be increased by coadministration with substrates or inhibitors of CYP1A2, such as imipramine, cimetidine, ciprofloxacin, fluvoxamine, isoniazid, ritonavir, theophylline, zileuton, zolmitriptan, and rizatriptan.
Substrates or Inhibitors of CYP2C19
Blood levels and/or toxicity of propranolol may be increased by coadministration with substrates or inhibitors of CYP2C19, such as fluconazole, cimetidine, fluoxetine, fluvoxamine, tenioposide, and tolbutamide. No interaction was observed with omeprazole.
Inducers of Hepatic Drug Metabolism
Blood levels of propranolol may be decreased by coadministration with inducers such as rifampin, ethanol, phenytoin, and phenobarbital. Cigarette smoking also induces hepatic metabolism and has been shown to increase up to 77% the clearance of propranolol, resulting in decreased plasma concentrations.
Cardiovascular Drugs
Antiarrhythmics
The AUC of propafenone is increased by more than 200% by coadministration of propranolol.
The metabolism of propranolol is reduced by coadministration of quinidine, leading to a two-three fold increased blood concentration and greater degrees of clinical beta-blockade.
The metabolism of lidocaine is inhibited by coadministration of propranolol, resulting in a 25% increase in lidocaine concentrations.
Calcium Channel Blockers
The mean Cmax and AUC of propranolol are increased, respectively, by 50% and 30% by coadministration of nisoldipine and by 80% and 47%, by coadministration of nicardipine.
The mean Cmax and AUC of nifedipine are increased by 64% and 79%, respectively, by coadministration of propranolol.
Propranolol does not affect the pharmacokinetics of verapamil and norverapamil. Verapamil does not affect the pharmacokinetics of propranolol.
Non-Cardiovascular Drugs
Migraine Drugs
Administration of zolmitriptan or rizatriptan with propranolol resulted in increased concentrations of zolmitriptan (AUC increased by 56% and Cmax by 37%) or rizatriptan (the AUC and Cmax were increased by 67% and 75%, respectively).
Theophylline
Coadministration of theophylline with propranolol decreases theophylline oral clearance by 30% to 52%.
Benzodiazepines
Propranolol can inhibit the metabolism of diazepam, resulting in increased concentrations of diazepam and its metabolites. Diazepam does not alter the pharmacokinetics of propranolol.
The pharmacokinetics of oxazepam, triazolam, lorazepam, and alprazolam are not affected by coadministration of propranolol.
Neuroleptic Drugs
Coadministration of long-acting propranolol at doses greater than or equal to 160 mg/day resulted in increased thioridazine plasma concentrations ranging from 55% to 369% and increased thioridazine metabolite (mesoridazine) concentrations ranging from 33% to 209%.
Coadministration of chlorpromazine with propranolol resulted in a 70% increase in propranolol plasma level.
Anti-Ulcer Drugs
Coadministration of propranolol with cimetidine, a non-specific CYP450 inhibitor, increased propranolol AUC and Cmax by 46% and 35%, respectively. Coadministration with aluminum hydroxide gel (1200 mg) may result in a decrease in propranolol concentrations.
Coadministration of metoclopramide with the long-acting propranolol did not have a significant effect on propranolol’s pharmacokinetics.
Lipid Lowering Drugs
Coadministration of cholestyramine or colestipol with propranolol resulted in up to 50% decrease in propranolol concentrations.
Coadministration of propranolol with lovastatin or pravastatin, decreased 18% to 23% the AUC of both, but did not alter their pharmacodynamics. Propranolol did not have an effect on the pharmacokinetics of fluvastatin.
Warfarin
Concomitant administration of propranolol and warfarin has been shown to increase warfarin bioavailability and increase prothrombin time.
Alcohol
Concomitant use of alcohol may increase plasma levels of propranolol.