Label: GOOD SENSE LANSOPRAZOLE- lansoprazole capsule, delayed release
- NDC Code(s): 0113-0117-01, 0113-0117-02, 0113-0117-03
- Packager: L. Perrigo Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 25, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
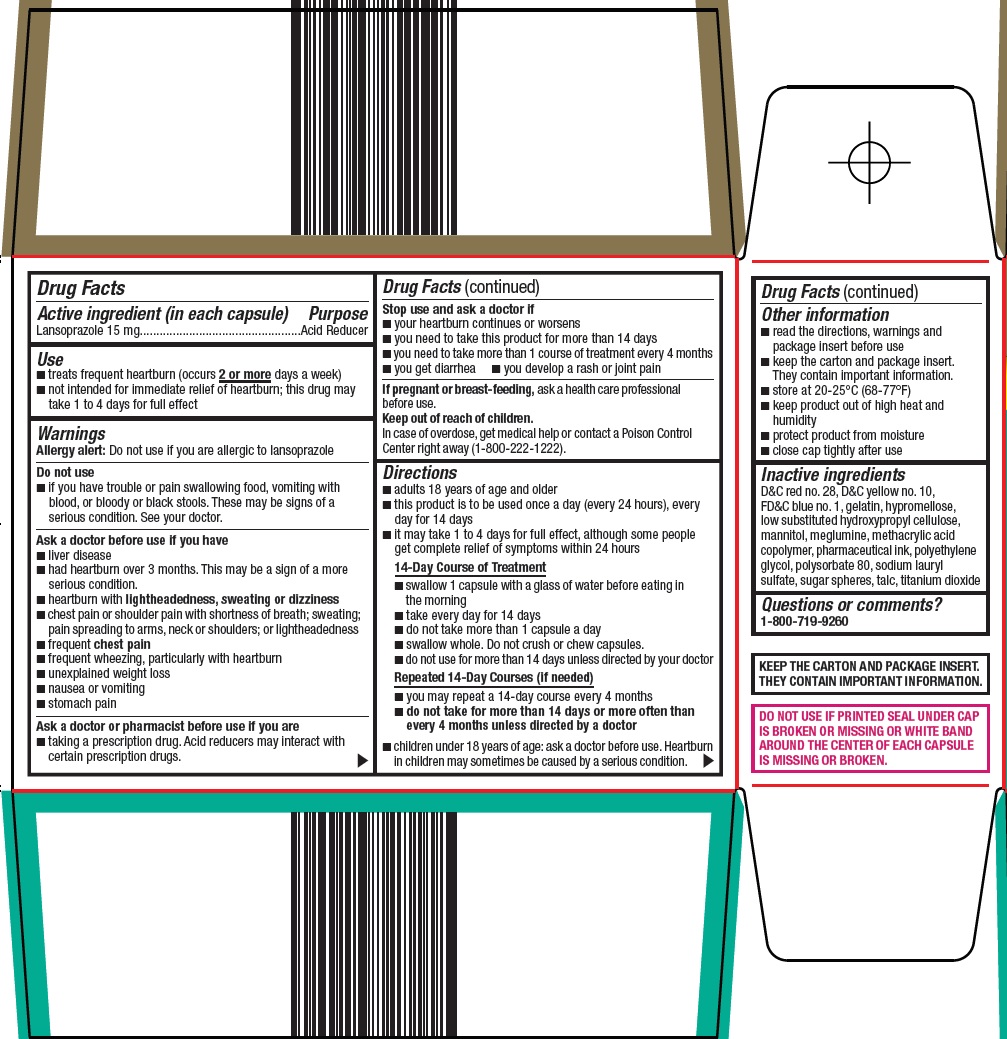
- Active ingredient (in each capsule)
- Purpose
- Use
-
Warnings
Allergy alert: Do not use if you are allergic to lansoprazole
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- •
- liver disease
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
Ask a doctor or pharmacist before use if you are
- •
- taking a prescription drug. Acid reducers may interact with certain prescription drugs.
-
Directions
- •
- adults 18 years of age and older
- •
- this product is to be used once a day (every 24 hours), every day for 14 days
- •
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- •
- swallow 1 capsule with a glass of water before eating in the morning
- •
- take every day for 14 days
- •
- do not take more than 1 capsule a day
- •
- swallow whole. Do not crush or chew capsules.
- •
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- •
- you may repeat a 14-day course every 4 months
- •
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- •
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- Other Information
- Inactive ingredients
- Questions or comments?
-
Consumer Information
Treats Frequent Heartburn
Lansoprazole
Delayed-Release Capsules 15 mg / Acid Reducer
- •
- May take 1 to 4 days for full effect
- •
- Sodium Free
Please read the entire package insert before taking Lansoprazole Delayed-Release Capsules 15 mg.
Save for future reference.
How Lansoprazole Delayed-Release Capsules 15 mg Treats Your Frequent Heartburn
Lansoprazole Delayed-Release Capsules 15 mg stops acid production at the source – the pumps that release acid into the stomach. Lansoprazole Delayed-Release Capsules 15 mg is taken once a day (every 24 hours), every day for 14 days.
What You Can Expect When Taking Lansoprazole Delayed-Release Capsules 15 mg
Frequent heartburn can occur anytime during the 24-hour period (day or night). Take Lansoprazole Delayed-Release Capsules 15 mg in the morning before eating. Lansoprazole Delayed-Release Capsules 15 mg is clinically proven to treat frequent heartburn. Although some people get complete relief of symptoms within 24 hours, it may take 1 to 4 days for full effect. Make sure you take Lansoprazole Delayed-Release Capsules 15 mg every day for 14 days to treat your frequent heartburn.
Who Should Take Lansoprazole Delayed-Release Capsules 15 mg
Adults (18 years and older) with frequent heartburn – when you have heartburn 2 or more days a week.
Who Should NOT Take Lansoprazole Delayed-Release Capsules 15 mg
People who have one episode of heartburn a week or less, or who want immediate relief of heartburn.
How to Take Lansoprazole Delayed-Release Capsules 15 mg
14-DAY Course of Treatment
- •
- Swallow 1 capsule with a glass of water before eating in the morning.
- •
- Take every day for 14 days.
- •
- Do not take more than 1 capsule a day.
- •
- Swallow whole. Do not crush or chew capsules.
- •
- Do not use for more than 14 days unless directed by your doctor.
When to Take Lansoprazole Delayed-Release Capsules 15 mg Again
You may repeat a 14-day course of therapy every 4 months.
When to Talk to Your Doctor
Do not take for more than 14 days or more often than every 4 months unless directed by a doctor.
Warnings and When to Ask Your Doctor
Allergy alert: Do not use if you are allergic to lansoprazole
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- •
- liver disease
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
Ask a doctor or pharmacist before use if you are
- •
- taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
- •
- your heartburn continues or worsens
- •
- you need to take this product for more than 14 days
- •
- you need to take more than 1 course of treatment every 4 months
- •
- you get diarrhea
- •
- you develop a rash or joint pain
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Tips for Managing Heartburn
- •
- Avoid foods or drinks that are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some acidic fruits and vegetables.
- •
- Eat slowly and do not eat big meals.
- •
- Do not eat late at night or just before bedtime.
- •
- Do not lie flat or bend over soon after eating.
- •
- Raise the head of your bed.
- •
- Wear loose-fitting clothing around your stomach.
- •
- If you are overweight, lose weight.
- •
- If you smoke, quit smoking.
Clinical studies prove Lansoprazole Delayed-Release Capsules 15 mg effectively treats frequent heartburn
In three clinical studies, Lansoprazole Delayed-Release Capsules 15 mg was shown to be significantly better than placebo in treating frequent heartburn.
How Lansoprazole Delayed-Release Capsules 15 mg is Sold
Lansoprazole Delayed-Release Capsules 15 mg is available in 14 capsule, 28 capsule and 42 capsule sizes. These sizes contain one, two and three 14-day courses of treatment, respectively. Do not use for more than 14 days in a row unless directed by your doctor. For the 28 count (two 14-day courses) and the 42 count (three 14-day courses), you may repeat a 14-day course every 4 months.
For Questions or Comments About Lansoprazole Delayed-Release Capsules 15 mg
Call 1-800-719-9260
Distributed By
Perrigo®
Allegan, MI 49010
: 3T300 00 J6
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GOOD SENSE LANSOPRAZOLE
lansoprazole capsule, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0113-0117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANSOPRAZOLE (UNII: 0K5C5T2QPG) (LANSOPRAZOLE - UNII:0K5C5T2QPG) LANSOPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) MANNITOL (UNII: 3OWL53L36A) MEGLUMINE (UNII: 6HG8UB2MUY) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK, GREEN Score no score Shape CAPSULE Size 16mm Flavor Imprint Code L3T3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0113-0117-01 1 in 1 CARTON 05/24/2012 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0113-0117-02 2 in 1 CARTON 05/24/2012 2 14 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:0113-0117-03 3 in 1 CARTON 05/24/2012 3 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202319 05/24/2012 Labeler - L. Perrigo Company (006013346)