Label: LANSOPRAZOLE capsule, delayed release
- NDC Code(s): 0093-7350-56, 0093-7351-56
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LANSOPRAZOLE DELAYED-RELEASE CAPSULES safely and effectively. See full prescribing information for LANSOPRAZOLE DELAYED-RELEASE ...These highlights do not include all the information needed to use LANSOPRAZOLE DELAYED-RELEASE CAPSULES safely and effectively. See full prescribing information for LANSOPRAZOLE DELAYED-RELEASE CAPSULES.
LANSOPRAZOLE delayed-release capsules, for oral use
Initial U.S. Approval: 1995RECENT MAJOR CHANGES
Warnings and Precautions,
Severe Cutaneous Adverse Reactions (5.5) 03/2022
Hypomagnesemia and Mineral Metabolism (5.8) 03/2022
INDICATIONS AND USAGE
Lansoprazole delayed-release capsules are a proton pump inhibitor (PPI) indicated for the:
• Treatment of active duodenal ulcer in adults. (1.1)
• Eradication of H. pylori to reduce the risk of duodenal ulcer recurrence in adults. (1.2)
• Maintenance of healed duodenal ulcers in adults. (1.3)
• Treatment of active benign gastric ulcer in adults. (1.4)
• Healing of nonsteroidal anti-inflammatory drugs (NSAID)-associated gastric ulcer in adults. (1.5)
• Risk reduction of NSAID-associated gastric ulcer in adults. (1.6)
• Treatment of symptomatic gastroesophageal reflux disease (GERD) in adults and pediatric patients 1 year of age and older. (1.7)
• Treatment of erosive esophagitis (EE) in adults and pediatric patients 1 year of age and older. (1.8)
• Maintenance of healing of EE in adults. (1.9)
• Pathological hypersecretory conditions, including Zollinger-Ellison syndrome (ZES) in adults. (1.10)
DOSAGE AND ADMINISTRATION
Recommended Dosage:
- See full prescribing information for complete dosing information for lansoprazole delayed-release capsules by indication and age group and dosage adjustment in patients with severe hepatic impairment. (2.1, 2.2, 2.3)
Administration Instructions (2.4)
Lansoprazole delayed-release capsules
- Should be swallowed whole.
- See full prescribing information for alternative administration options.
DOSAGE FORMS AND STRENGTHS
- Delayed- release capsules: 15 mg and 30 mg. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Gastric Malignancy: In adults, symptomatic response with lansoprazole does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing. (5.1)
- Acute Tubulointerstitial Nephritis: Discontinue treatment and evaluate patients. (5.2)
- Clostridium difficile-Associated Diarrhea: PPI therapy may be associated with increased risk of Clostridium difficile-associated diarrhea. (5.3)
- Bone Fracture: Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.4)
- Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
- Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue lansoprazole and refer to specialist for evaluation. (5.6)
- Cyanocobalamin (Vitamin B12) Deficiency: Daily long-term use (e.g., longer than 3 years) may lead to malabsorption or a deficiency of cyanocobalamin. (5.7)
- Hypomagnesemia and Mineral Metabolism: Hypomagnesemia has been reported rarely with prolonged treatment with PPIs. (5.8)
- Interactions with Investigations for Neuroendocrine Tumors: Increases in intragastric pH may result in hypergastrinemia and enterochromaffin-like cell hyperplasia and increased chromogranin A levels which may interfere with diagnostic investigations for neuroendocrine tumors. (5.9, 7)
- Interaction with Methotrexate: Concomitant use with PPIs may elevate and/or prolong serum concentrations of methotrexate and/or its metabolite, possibly leading to toxicity. With high-dose methotrexate administration, consider a temporary withdrawal of lansoprazole delayed-release capsules. (5.10, 7)
- Fundic Gland Polyps: Risk increases with long-term use, especially beyond 1 year. Use the shortest duration of therapy. (5.12)
- Risk of Heart Valve Thickening in Pediatric Patients Less than One Year of Age: Lansoprazole delayed-release capsule is not recommended in pediatric patients less than 1 year of age. (5.13, 8.4)
ADVERSE REACTIONS
Most commonly reported adverse reactions (≥1%): diarrhea, abdominal pain, nausea and constipation. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See full prescribing information for a list of clinically important drug interactions. (7)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause adverse effects on fetal bone growth and development. (8.1)
- Pediatrics: Use is not recommended for the treatment of symptomatic GERD in patients 1 month to less than 1 year of age; efficacy was not demonstrated and nonclinical studies have demonstrated adverse effects in juvenile rats. (5.13, 8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2022
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Active Duodenal Ulcer
1.2 Eradication of H. pylori to Reduce the Risk of Duodenal Ulcer Recurrence
1.3 Maintenance of Healed Duodenal Ulcers
1.4 Treatment of Active Benign Gastric Ulcer
1.5 Healing of NSAID-Associated Gastric Ulcer
1.6 Risk Reduction of NSAID-Associated Gastric Ulcer
1.7 Treatment of Symptomatic Gastroesophageal Reflux Disease (GERD)
1.8 Treatment of Erosive Esophagitis (EE)
1.9 Maintenance of Healing of EE
1.10 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome (ZES)
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Adult Dosage by Indication
2.2 Recommended Pediatric Dosage by Indication
2.3 Hepatic Impairment
2.4 Important Administration Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
5.2 Acute Tubulointerstitial Nephritis
5.3 Clostridium difficile-Associated Diarrhea
5.4 Bone Fracture
5.5 Severe Cutaneous Adverse Reactions
5.6 Cutaneous and Systemic Lupus Erythematosus
5.7 Cyanocobalamin (Vitamin B12) Deficiency
5.8 Hypomagnesemia and Mineral Metabolism
5.9 Interactions with Investigations for Neuroendocrine Tumors
5.10 Interaction with Methotrexate
5.12 Fundic Gland Polyps
5.13 Risk of Heart Valve Thickening in Pediatric Patients Less Than One Year of Age
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Combination Therapy with Amoxicillin and Clarithromycin
6.4 Laboratory Values
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Duodenal Ulcer
14.2 Eradication of H. pylori to Reduce the Risk of Duodenal Ulcer Recurrence
14.3 Maintenance of Healed Duodenal Ulcers
14.4 Gastric Ulcer
14.5 Healing of NSAID-Associated Gastric Ulcer
14.6 Risk Reduction of NSAID-Associated Gastric Ulcer
14.7 Symptomatic Gastroesophageal Reflux Disease (GERD)
14.8 Erosive Esophagitis
14.9 Maintenance of Healing of Erosive Esophagitis
14.10 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Treatment of Active Duodenal Ulcer - Lansoprazole delayed-release capsules are indicated in adults for short-term treatment (for four weeks) for healing and symptom relief of active duodenal ...
1.1 Treatment of Active Duodenal Ulcer
Lansoprazole delayed-release capsules are indicated in adults for short-term treatment (for four weeks) for healing and symptom relief of active duodenal ulcer [see Clinical Studies (14.1)].
1.2 Eradication of H. pylori to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy: Lansoprazole delayed-release capsules/amoxicillin/clarithromycin
Lansoprazole delayed-release capsules in combination with amoxicillin plus clarithromycin as triple therapy is indicated in adults for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one year history of a duodenal ulcer) to eradicate H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence [see Clinical Studies (14.2)].
Please refer to the full prescribing information for amoxicillin and clarithromycin.
Dual Therapy: Lansoprazole delayed-release capsules/amoxicillin
Lansoprazole delayed-release capsules in combination with amoxicillin as dual therapy is indicated in adults for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one year history of a duodenal ulcer) who are either allergic or intolerant to clarithromycin or in whom resistance to clarithromycin is known or suspected (see the clarithromycin prescribing information, Microbiology section). Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence [see Clinical Studies (14.2)].
Please refer to the full prescribing information for amoxicillin.
1.3 Maintenance of Healed Duodenal Ulcers
Lansoprazole delayed-release capsules are indicated in adults to maintain healing of duodenal ulcers. Controlled studies do not extend beyond 12 months [see Clinical Studies (14.3)].
1.4 Treatment of Active Benign Gastric Ulcer
Lansoprazole delayed-release capsules are indicated in adults for short-term treatment (up to eight weeks) for healing and symptom relief of active benign gastric ulcer [see Clinical Studies (14.4)].
1.5 Healing of NSAID-Associated Gastric Ulcer
Lansoprazole delayed-release capsules are indicated in adults for the treatment of NSAID-associated gastric ulcer in patients who continue NSAID use. Controlled studies did not extend beyond eight weeks [see Clinical Studies (14.5)].
1.6 Risk Reduction of NSAID-Associated Gastric Ulcer
Lansoprazole delayed-release capsules are indicated in adults for reducing the risk of NSAID-associated gastric ulcers in patients with a history of a documented gastric ulcer who require the use of an NSAID. Controlled studies did not extend beyond 12 weeks [see Clinical Studies (14.6)].
1.7 Treatment of Symptomatic Gastroesophageal Reflux Disease (GERD)
Lansoprazole delayed-release capsules are indicated for short-term treatment in adults and pediatric patients 12 to 17 years of age (up to eight weeks) and pediatric patients one to 11 years of age (up to 12 weeks) for the treatment of heartburn and other symptoms associated with GERD [see Clinical Studies (14.7)].
1.8 Treatment of Erosive Esophagitis (EE)
Lansoprazole delayed-release capsules are indicated for short-term treatment in adults and pediatric patients 12 to 17 years of age (up to eight weeks) and pediatric patients one to 11 years of age (up to 12 weeks) for healing and symptom relief of all grades of EE.
For adults who do not heal with lansoprazole delayed-release capsules for eight weeks (5 to 10%), it may be helpful to give an additional eight weeks of treatment. If there is a recurrence of erosive esophagitis an additional eight week course of lansoprazole delayed-release capsules may be considered [see Clinical Studies (14.8)].
1.9 Maintenance of Healing of EE
Lansoprazole delayed-release capsules are indicated in adults to maintain healing of EE. Controlled studies did not extend beyond 12 months [see Clinical Studies (14.9)].
Close1.10 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome (ZES)
Lansoprazole delayed-release capsules are indicated in adults for the long-term treatment of pathological hypersecretory conditions, including Zollinger-Ellison syndrome [see Clinical Studies (14.10)].
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Adult Dosage by Indication - Indication - Recommended Dose - Frequency - Duodenal Ulcers - Short-Term Treatment - 15 mg - Once daily for 4 weeks ...
2.1 Recommended Adult Dosage by Indication
Indication
Recommended Dose
Frequency
Duodenal Ulcers
Short-Term Treatment
15 mg
Once daily for 4 weeks
Maintenance of Healed
15 mg
Once daily
Eradication of H. pylori to Reduce the Risk of Duodenal Ulcer Recurrence*
Triple Therapy:
Lansoprazole
30 mg
Twice daily for 10 or 14 days
Amoxicillin
1 gram
Twice daily for 10 or 14 days
Clarithromycin
500 mg
Twice daily for 10 or 14 days
Dual Therapy:
Lansoprazole
30 mg
Three times daily for 14 days
Amoxicillin
1 gram
Three times daily for 14 days
Benign Gastric Ulcer
Short-Term Treatment
30 mg
Once daily for up to 8 weeks
NSAID-Associated Gastric Ulcer
Healing
30 mg
Once daily for 8 weeks†
Risk Reduction
15 mg
Once daily for up to 12 weeks†
Gastroesophageal Reflux Disease (GERD)
Short-Term Treatment of Symptomatic GERD
15 mg
Once daily for up to 8 weeks
Short-Term Treatment of Erosive Esophagitis
30 mg
Once daily for up to 8 weeks‡
Maintenance of Healing of Erosive Esophagitis
15 mg
Once daily¶
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
60 mg
Once daily§
*Please refer to the amoxicillin and clarithromycin full prescribing information, Contraindications and Warnings and Precautions sections, and for information regarding dosing in elderly and renally-impaired patients.
† Controlled studies did not extend beyond indicated duration.
‡ For patients who do not heal with lansoprazole delayed-release capsules for eight weeks (5 to 10%), it may be helpful to give an additional eight weeks of treatment. If there is a recurrence of erosive esophagitis, an additional eight week course of lansoprazole delayed-release capsules may be considered.
§ Varies with individual patient. Recommended adult starting dose is 60 mg once daily. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Dosages up to 90 mg twice daily have been administered. Daily dose of greater than 120 mg should be administered in divided doses. Some patients with Zollinger-Ellison syndrome have been treated continuously with lansoprazole for more than four years.
¶ Controlled studies did not extend beyond 12 months.
2.2 Recommended Pediatric Dosage by Indication
Pediatric Patients 1 to 11 Years of Age
In clinical studies, lansoprazole delayed-release capsules were not administered beyond 12 weeks in 1 to 11 year olds. It is not known if lansoprazole delayed-release capsules are safe and effective if used longer than the recommended duration. Do not exceed the recommended dose and duration of use in pediatric patients as outlined below [see Use in Specific Populations (8.4)].
Indication
Recommended Dose
Frequency
Short-Term Treatment of Symptomatic GERD and Short-Term Treatment of Erosive Esophagitis
≤ 30 kg
15 mg
Once daily for up to 12 weeks
> 30 kg
30 mg
Once daily for up to 12 weeks
Pediatric Patients 12 to 17 Years of Age
Indication
Recommended Dose
Frequency
Short-Term Treatment of Symptomatic GERD
Non-erosive GERD
15 mg
Once daily for up to 8 weeks
Erosive Esophagitis
30 mg
Once daily for up to 8 weeks
2.3 Hepatic Impairment
The recommended dosage is 15 mg orally daily in patients with severe liver impairment (Child-Pugh C) [see Use in Specific Populations (8.6)].
Close2.4 Important Administration Information
- Take lansoprazole delayed-release capsules before meals.
- Do not crush or chew lansoprazole delayed-release capsules.
- Take lansoprazole delayed-release capsules at least 30 minutes prior to sucralfate [see Drug Interactions (7)].
- Antacids may be used concomitantly with lansoprazole delayed-release capsules.
- Missed doses: If a dose is missed, administer as soon as possible. However, if the next scheduled dose is due, do not take the missed dose, and take the next dose on time. Do not take two doses at one time to make up for a missed dose.
Lansoprazole Delayed-Release Capsules - Swallow whole; do not chew.
- For patients who have difficulty swallowing capsules, lansoprazole delayed-release capsules can be opened and administered orally or via a nasogastric tube in the soft foods or liquids specified below.
- Administration of lansoprazole delayed-release capsules in foods or liquids other than those discussed below have not been studied clinically and therefore are not recommended.
Administration in Soft Foods (applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears):
- Open capsule.
- Sprinkle intact granules on one tablespoon of either applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
- Swallow immediately.
Administration in Liquids (apple juice, orange juice or tomato juice):
- Open capsule.
- Sprinkle intact granules into a small volume of either apple juice, orange juice or tomato juice (60 mL – approximately two ounces).
- Mix briefly.
- Swallow immediately.
- To ensure complete delivery of the dose, rinse the glass with two or more volumes of juice and swallow the contents immediately.
Administration with Apple Juice Through a Nasogastric Tube (≥16 French)
- Open capsule.
- Sprinkle intact granules into 40 mL of apple juice.
- Mix briefly.
- Using a catheter-tipped syringe, draw up the mixture.
- Inject through the nasogastric tube into the stomach.
- Flush with additional apple juice to clear the tube.
-
3 DOSAGE FORMS AND STRENGTHS15 mg are hard gelatin capsules, with a light-blue opaque cap and flesh-colored opaque body, imprinted with “93” and “7350”. 30 mg are hard gelatin capsules, with a light-gray opaque cap and ...
- 15 mg are hard gelatin capsules, with a light-blue opaque cap and flesh-colored opaque body, imprinted with “93” and “7350”.
- 30 mg are hard gelatin capsules, with a light-gray opaque cap and flesh-colored opaque body, imprinted with “93” and “7351”.
-
4 CONTRAINDICATIONSLansoprazole delayed-release capsules are contraindicated in patients with known hypersensitivity to any component of the formulation. Hypersensitivity reactions may include anaphylaxis ...
- Lansoprazole delayed-release capsules are contraindicated in patients with known hypersensitivity to any component of the formulation. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute tubulointerstitial nephritis, and urticaria [see Warnings and Precautions (5.2), Adverse Reactions (6)].
- Proton Pump Inhibitors (PPIs), including lansoprazole delayed-release capsules, are contraindicated with rilpivirine-containing products [see Drug Interactions (7)].
- For information about contraindications of antibacterial agents (clarithromycin and amoxicillin) indicated in combination with lansoprazole delayed-release capsules, refer to the Contraindications section of their prescribing information.
-
5 WARNINGS AND PRECAUTIONS5.1 Presence of Gastric Malignancy - In adults, symptomatic response to therapy with lansoprazole does not preclude the presence of gastric malignancy. Consider additional follow-up and ...
5.1 Presence of Gastric Malignancy
In adults, symptomatic response to therapy with lansoprazole does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing in adult patients who have a suboptimal response or an early symptomatic relapse after completing treatment with a PPI. In older patients, also consider an endoscopy.
5.2 Acute Tubulointerstitial Nephritis
Acute tubulointerstitial nephritis (TIN) has been observed in patients taking PPIs and may occur at any point during PPI therapy. Patients may present with varying signs and symptoms from symptomatic hypersensitivity reactions to non-specific symptoms of decreased renal function (e.g., malaise, nausea, anorexia). In reported case series, some patients were diagnosed on biopsy and in the absence of extra-renal manifestations (e.g., fever, rash or arthralgia). Discontinue lansoprazole delayed-release capsules and evaluate patients with suspected acute TIN [see Contraindications (4)].
5.3 Clostridium difficile-Associated Diarrhea
Published observational studies suggest that PPI therapy like lansoprazole delayed-release capsules may be associated with an increased risk of Clostridium difficile-associated diarrhea (CDAD), especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve [see Adverse Reactions (6.2)].
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
CDAD has been reported with use of nearly all antibacterial agents. For more information specific to antibacterial agents (clarithromycin and amoxicillin) indicated for use in combination with lansoprazole delayed-release capsules, refer to Warnings and Precautions section of their prescribing information.
5.4 Bone Fracture
Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines [see Dosage and Administration (2), Adverse Reactions (6.2)].
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in association with the use of PPIs [see Adverse Reactions (6.2)]. Discontinue lansoprazole delayed-release capsules at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Cutaneous and Systemic Lupus Erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including lansoprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematosus cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI-associated SLE is usually milder than nondrug induced SLE. Onset of SLE typically occurred within days to years after initiating treatment primarily in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Avoid administration of PPIs for longer than medically indicated. If signs or symptoms consistent with CLE or SLE are noted in patients receiving lansoprazole, discontinue the drug and refer the patient to the appropriate specialist for evaluation. Most patients improve with discontinuation of the PPI alone in four to 12 weeks. Serological testing (e.g., ANA) may be positive and elevated serological test results may take longer to resolve than clinical manifestations.
5.7 Cyanocobalamin (Vitamin B12) Deficiency
Daily treatment with any acid-suppressing medications over a long period of time (e.g., longer than three years) may lead to malabsorption of cyanocobalamin (Vitamin B12) caused by hypo- or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed in patients treated with lansoprazole.
5.8 Hypomagnesemia and Mineral Metabolism
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. Hypomagnesemia may lead to hypocalcemia and/or hypokalemia and may exacerbate underlying hypocalcemia in at-risk patients. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically [see Adverse Reactions (6.2)].
Consider monitoring magnesium and calcium levels prior to initiation of lansoprazole delayed-release capsules and periodically while on treatment in patients with a preexisting risk of hypocalcemia (e.g., hypoparathyroidism).Supplement with magnesium and/or calcium, as necessary. If hypocalcemia is refractory to treatment, consider discontinuing the PPI.
5.9 Interactions with Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Healthcare providers should temporarily stop lansoprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary [see Drug Interactions (7), Clinical Pharmacology (12.2)].
5.10 Interaction with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients [see Drug Interactions (7), Clinical Pharmacology (12.3)].
5.12 Fundic Gland Polyps
PPI use is associated with an increased risk of fundic gland polyps that increases with long-term use, especially beyond one year. Most PPI users who developed fundic gland polyps were asymptomatic and fundic gland polyps were identified incidentally on endoscopy. Use the shortest duration of PPI therapy appropriate to the condition being treated.
Close5.13 Risk of Heart Valve Thickening in Pediatric Patients Less Than One Year of Age
Lansoprazole is not approved in pediatric patients less than one year of age. Nonclinical studies in juvenile rats with lansoprazole have demonstrated an adverse effect of heart valve thickening. The risk of heart valve injury does not appear to be relevant to patients one year of age and older [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in labeling: Acute Tubulointerstitial Nephritis [see Warnings and Precautions (5.2)] Clostridium difficile-Associated ...
The following serious adverse reactions are described below and elsewhere in labeling:
- Acute Tubulointerstitial Nephritis [see Warnings and Precautions (5.2)]
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.3)]
- Bone Fracture [see Warnings and Precautions (5.4)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.5)]
- Cutaneous and Systemic Lupus Erythematosus [see Warnings and Precautions (5.6)]
- Cyanocobalamin (Vitamin B12) Deficiency [see Warnings and Precautions (5.7)]
- Hypomagnesemia and Mineral Metabolism [see Warnings and Precautions (5.8)]
- Fundic Gland Polyps [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Worldwide, over 10,000 patients have been treated with lansoprazole in Phase 2 or Phase 3 clinical trials involving various dosages and durations of treatment. In general, lansoprazole treatment has been well-tolerated in both short-term and long-term trials.
The following adverse reactions were reported by the treating physician to have a possible or probable relationship to drug in 1% or more of lansoprazole-treated patients and occurred at a greater rate in lansoprazole-treated patients than placebo-treated patients in Table 1.
Table 1. Incidence of Possibly or Probably Treatment-Related Adverse Reactions in Short-Term, Placebo-Controlled Lansoprazole Studies Body System/
Adverse ReactionLansoprazole
Placebo
(N=2768)
(N=1023)
%
%
Body as a Whole
Abdominal Pain
2.1
1.2
Digestive System
Constipation
1.0
0.4
Diarrhea
3.8
2.3
Nausea
1.3
1.2
Headache was also seen at greater than 1% incidence but was more common on placebo. The incidence of diarrhea was similar between patients who received placebo and patients who received 15 and 30 mg of lansoprazole, but higher in the patients who received 60 mg of lansoprazole (2.9, 1.4, 4.2, and 7.4%, respectively).The most commonly reported possibly or probably treatment-related adverse event during maintenance therapy was diarrhea.
In the risk reduction study of lansoprazole for NSAID-associated gastric ulcers, the incidence of diarrhea for patients treated with lansoprazole, misoprostol, and placebo was 5, 22, and 3%, respectively.
Another study for the same indication, where patients took either a COX-2 inhibitor or lansoprazole and naproxen, demonstrated that the safety profile was similar to the prior study. Additional reactions from this study not previously observed in other clinical trials with lansoprazole included contusion, duodenitis, epigastric discomfort, esophageal disorder, fatigue, hunger, hiatal hernia, hoarseness, impaired gastric emptying, metaplasia, and renal impairment.
Additional adverse experiences occurring in less than 1% of patients or subjects who received lansoprazole in domestic trials are shown below:
Body as a Whole - abdomen enlarged, allergic reaction, asthenia, back pain, candidiasis, carcinoma, chest pain (not otherwise specified), chills, edema, fever, flu syndrome, halitosis, infection (not otherwise specified), malaise, neck pain, neck rigidity, pain, pelvic pain
Cardiovascular System - angina, arrhythmia, bradycardia, cerebrovascular accident/cerebral infarction, hypertension/hypotension, migraine, myocardial infarction, palpitations, shock (circulatory failure), syncope, tachycardia, vasodilation
Digestive System - abnormal stools, anorexia, bezoar, cardiospasm, cholelithiasis, colitis, dry mouth, dyspepsia, dysphagia, enteritis, eructation, esophageal stenosis, esophageal ulcer, esophagitis, fecal discoloration, flatulence, gastric nodules/fundic gland polyps, gastritis, gastroenteritis, gastrointestinal anomaly, gastrointestinal disorder, gastrointestinal hemorrhage, glossitis, gum hemorrhage, hematemesis, increased appetite, increased salivation, melena, mouth ulceration, nausea and vomiting, nausea and vomiting and diarrhea, gastrointestinal moniliasis, rectal disorder, rectal hemorrhage, stomatitis, tenesmus, thirst, tongue disorder, ulcerative colitis, ulcerative stomatitis
Endocrine System - diabetes mellitus, goiter, hypothyroidism
Hemic and Lymphatic System - anemia, hemolysis, lymphadenopathy
Metabolism and Nutritional Disorders - avitaminosis, gout, dehydration, hyperglycemia/hypoglycemia, peripheral edema, weight gain/loss
Musculoskeletal System - arthralgia, arthritis, bone disorder, joint disorder, leg cramps, musculoskeletal pain, myalgia, myasthenia, ptosis, synovitis
Nervous System - abnormal dreams, agitation, amnesia, anxiety, apathy, confusion, convulsion, dementia, depersonalization, depression, diplopia, dizziness, emotional lability, hallucinations, hemiplegia, hostility aggravated, hyperkinesia, hypertonia, hypesthesia, insomnia, libido decreased/increased, nervousness, neurosis, paresthesia, sleep disorder, somnolence, thinking abnormality, tremor, vertigo
Respiratory System - asthma, bronchitis, cough increased, dyspnea, epistaxis, hemoptysis, hiccup, laryngeal neoplasia, lung fibrosis, pharyngitis, pleural disorder, pneumonia, respiratory disorder, upper respiratory inflammation/infection, rhinitis, sinusitis, stridor
Skin and Appendages - acne, alopecia, contact dermatitis, dry skin, fixed eruption, hair disorder, maculopapular rash, nail disorder, pruritus, rash, skin carcinoma, skin disorder, sweating, urticaria
Special Senses - abnormal vision, amblyopia, blepharitis, blurred vision, cataract, conjunctivitis, deafness, dry eyes, ear/eye disorder, eye pain, glaucoma, otitis media, parosmia, photophobia, retinal degeneration/disorder, taste loss, taste perversion, tinnitus, visual field defect
Urogenital System - abnormal menses, breast enlargement, breast pain, breast tenderness, dysmenorrhea, dysuria, gynecomastia, impotence, kidney calculus, kidney pain, leukorrhea, menorrhagia, menstrual disorder, penis disorder, polyuria, testis disorder, urethral pain, urinary frequency, urinary retention, urinary tract infection, urinary urgency, urination impaired, vaginitis.
6.2 Postmarketing Experience
Additional adverse experiences have been reported since lansoprazole has been marketed. The majority of these cases are foreign-sourced and a relationship to lansoprazole has not been established. Because these reactions were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events are listed below by COSTART body system.
Body as a Whole – anaphylactic/anaphylactoid reactions, systemic lupus erythematosus; Digestive System – hepatotoxicity, pancreatitis, vomiting; Hemic and Lymphatic System – agranulocytosis, aplastic anemia, hemolytic anemia, leukopenia, neutropenia, pancytopenia, thrombocytopenia, and thrombotic thrombocytopenic purpura; Infections and Infestations – Clostridium difficile-associated diarrhea; Metabolism and Nutritional Disorders – hypomagnesemia, hypocalcemia, hypokalemia, hyponatremia; Musculoskeletal System – bone fracture, myositis; Skin and Appendages – severe dermatologic reactions including erythema multiforme, SJS/TEN (some fatal), DRESS, AGEP, cutaneous lupus erythematosus; Special Senses – speech disorder; Urogenital System – interstitial nephritis, urinary retention.
6.3 Combination Therapy with Amoxicillin and Clarithromycin
In clinical trials using combination therapy with lansoprazole plus amoxicillin and clarithromycin, and lansoprazole plus amoxicillin, no adverse reactions peculiar to these drug combinations were observed. Adverse reactions that have occurred have been limited to those that had been previously reported with lansoprazole, amoxicillin, or clarithromycin.
Triple Therapy: Lansoprazole/amoxicillin/clarithromycin
The most frequently reported adverse reactions for patients who received triple therapy for 14 days were diarrhea (7%), headache (6%), and taste perversion (5%). There were no statistically significant differences in the frequency of reported adverse reactions between the 10 and 14 day triple therapy regimens. No treatment-emergent adverse reactions were observed at significantly higher rates with triple therapy than with any dual therapy regimen.
Dual Therapy: Lansoprazole/amoxicillin
The most frequently reported adverse reactions for patients who received lansoprazole three times daily plus amoxicillin three times daily dual therapy were diarrhea (8%) and headache (7%). No treatment-emergent adverse reactions were observed at significantly higher rates with lansoprazole three times daily plus amoxicillin three times daily dual therapy than with lansoprazole alone.
For information about adverse reactions with antibacterial agents (amoxicillin and clarithromycin) indicated in combination with lansoprazole, refer to the Adverse Reactions section of their prescribing information.
Close6.4 Laboratory Values
The following changes in laboratory parameters in patients who received lansoprazole were reported as adverse reactions:
Abnormal liver function tests, increased SGOT (AST), increased SGPT (ALT), increased creatinine, increased alkaline phosphatase, increased globulins, increased GGTP, increased/decreased/abnormal WBC, abnormal AG ratio, abnormal RBC, bilirubinemia, blood potassium increased, blood urea increased, crystal urine present, eosinophilia, hemoglobin decreased, hyperlipemia, increased/decreased electrolytes, increased/decreased cholesterol, increased glucocorticoids, increased LDH, increased/decreased/abnormal platelets, increased gastrin levels and positive fecal occult blood. Urine abnormalities such as albuminuria, glycosuria, and hematuria were also reported. Additional isolated laboratory abnormalities were reported.
In the placebo-controlled studies, when SGOT (AST) and SGPT (ALT) were evaluated, 0.4% (4/978) and 0.4% (11/2677) patients, who received placebo and lansoprazole, respectively, had enzyme elevations greater than three times the upper limit of normal range at the final treatment visit. None of these patients who received lansoprazole reported jaundice at any time during the study.
In clinical trials using combination therapy with lansoprazole plus amoxicillin and clarithromycin, and lansoprazole plus amoxicillin, no increased laboratory abnormalities particular to these drug combinations were observed.
For information about laboratory value changes with antibacterial agents (amoxicillin and clarithromycin) indicated in combination with lansoprazole, refer to the Adverse Reactions section of their prescribing information.
-
7 DRUG INTERACTIONSTables 2 and 3 include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with lansoprazole and instructions for preventing or ...
Tables 2 and 3 include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with lansoprazole and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
Table 2. Clinically Relevant Interactions Affecting Drugs Coadministered with Lansoprazole and Interactions with Diagnostics Antiretrovirals
Clinical Impact:
The effect of PPIs on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir, and nelfinavir) when used concomitantly with lansoprazole may reduce antiviral effect and promote the development of drug resistance.
- Increased exposure of other antiretroviral drugs (e.g., saquinavir) when used concomitantly with lansoprazole may increase toxicity of the antiretroviral drugs.
- There are other antiretroviral drugs which do not result in clinically relevant interactions with lansoprazole.
Intervention:
Rilpivirine-containing products: Concomitant use with lansoprazole is contraindicated [see Contraindications (4)]. See prescribing information.
Atazanavir: See prescribing information for atazanavir for dosing information.
Nelfinavir: Avoid concomitant use with lansoprazole. See prescribing information for nelfinavir.
Saquinavir: See the prescribing information for saquinavir and monitor for potential saquinavir toxicities.
Other antiretrovirals: See prescribing information.
Warfarin
Clinical Impact:
Increased INR and prothrombin time in patients receiving PPIs and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Intervention:
Monitor INR and prothrombin time. Dose adjustment of warfarin may be needed to maintain target INR range. See prescribing information for warfarin.
Methotrexate
Clinical Impact:
Concomitant use of PPIs with methotrexate (primarily at high dose) may elevate and prolong serum concentrations of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. No formal drug interaction studies of high-dose methotrexate with PPIs have been conducted [see Warnings and Precautions (5.10)].
Intervention:
A temporary withdrawal of lansoprazole may be considered in some patients receiving high-dose methotrexate.
Digoxin
Clinical Impact:
Potential for increased exposure of digoxin.
Intervention:
Monitor digoxin concentrations. Dose adjustment of digoxin may be needed to maintain therapeutic drug concentrations. See prescribing information for digoxin.
Theophylline
Clinical Impact:
Increased clearance of theophylline [see Clinical Pharmacology (12.3)].
Intervention:
Individual patients may require additional titration of their theophylline dosage when lansoprazole is started or stopped to ensure clinically effective blood concentrations.
Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole)
Clinical Impact:
Lansoprazole can reduce the absorption of other drugs due to its effect on reducing intragastric acidity.
Intervention:
Mycophenolate mofetil (MMF): Coadministration of PPIs in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving lansoprazole and MMF. Use lansoprazole with caution in transplant patients receiving MMF.
See the prescribing information for other drugs dependent on gastric pH for absorption.
Combination Therapy with Clarithromycin and Amoxicillin
Clinical Impact:
Concomitant administration of clarithromycin with other drugs can lead to serious adverse reactions, including potentially fatal arrhythmias, and are contraindicated. Amoxicillin also has drug interactions.
Intervention:
- See Contraindications and Warnings and Precautions in prescribing information for clarithromycin.
- See Drug Interactions in prescribing information for amoxicillin.
Tacrolimus
Clinical Impact:
Potentially increased exposure of tacrolimus, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19.
Intervention:
Monitor tacrolimus whole blood trough concentrations. Dose adjustment of tacrolimus may be needed to maintain therapeutic drug concentrations. See prescribing information for tacrolimus.
Interactions with Investigations of Neuroendocrine Tumors
Clinical Impact:
CgA levels increase secondary to PPI-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions (5.9), Clinical Pharmacology (12.2)].
Intervention:
Temporarily stop lansoprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
Interaction with Secretin Stimulation Test
Clinical Impact:
Hyper-response in gastrin secretion in response to secretin stimulation test, falsely suggesting gastrinoma.
Intervention:
Temporarily stop lansoprazole treatment at least 28 days before assessing to allow gastrin levels to return to baseline [see Clinical Pharmacology (12.2)].
False Positive Urine Tests for THC
Clinical Impact:
There have been reports of false positive urine screening tests for tetrahydrocannabinol (THC) in patients receiving PPIs.
Intervention:
An alternative confirmatory method should be considered to verify positive results.
CloseTable 3. Clinically Relevant Interactions Affecting Lansoprazole When Coadministered with Other Drugs CYP2C19 OR CYP3A4 Inducers
Clinical Impact:
Decreased exposure of lansoprazole when used concomitantly with strong inducers [see Clinical Pharmacology (12.3)].
Intervention:
St. John’s Wort, rifampin: Avoid concomitant use with lansoprazole.
Ritonavir-containing products: See prescribing information.
CYP2C19 or CYP3A4 Inhibitors
Clinical Impact:
Increased exposure of lansoprazole is expected when used concomitantly with strong inhibitors [see Clinical Pharmacology (12.3)].
Intervention:
Voriconazole: See prescribing information.
Sucralfate
Clinical Impact:
Decreased and delayed absorption of lansoprazole [see Clinical Pharmacology (12.3)].
Intervention:
Take lansoprazole at least 30 minutes prior to sucralfate [see Dosage and Administration (2.4)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published observational studies overall do not indicate an association of adverse pregnancy outcomes with lansoprazole treatment (see Data) ...
8.1 Pregnancy
Risk Summary
Available data from published observational studies overall do not indicate an association of adverse pregnancy outcomes with lansoprazole treatment (see Data).
In animal reproduction studies, oral administration of lansoprazole to rats during organogenesis through lactation at 6.4 times the maximum recommended human dose produced reductions in the offspring in femur weight, femur length, crown-rump length and growth plate thickness (males only) on postnatal Day 21 (see Data). These effects were associated with reduction in body weight gain. Advise pregnant women of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
If lansoprazole delayed-release capsules are administered with clarithromycin, the pregnancy information for clarithromycin also applies to the combination regimen. Refer to the prescribing information for clarithromycin for more information on use in pregnancy.
Data
Human Data
Available data from published observational studies failed to demonstrate an association of adverse pregnancy-related outcomes and lansoprazole use. Methodological limitations of these observational studies cannot definitely establish or exclude any drug-associated risk during pregnancy. In a prospective study by the European Network of Teratology Information Services, outcomes from a group of 62 pregnant women administered median daily doses of 30 mg of lansoprazole were compared to a control group of 868 pregnant women who did not take any PPIs. There was no difference in the rate of major malformations between women exposed to PPIs and the control group, corresponding to a Relative Risk (RR)=1.04, [95% Confidence Interval (CI) 0.25 to 4.21]. In a population-based retrospective cohort study covering all live births in Denmark from 1996 to 2008, there was no significant increase in major birth defects during analysis of first trimester exposure to lansoprazole in 794 live births. A meta-analysis that compared 1,530 pregnant women exposed to PPIs in at least the first trimester with 133,410 unexposed pregnant women showed no significant increases in risk for congenital malformations or spontaneous abortion with exposure to PPIs (for major malformations Odds Ratio (OR)=1.12, [95% CI 0.86 to 1.45] and for spontaneous abortions OR=1.29, [95% CI 0.84 to 1.97]).
Animal Data
No adverse effects on embryo-fetal development occurred in studies performed in pregnant rats at oral lansoprazole doses up to 150 mg/kg/day (40 times the recommended human dose [30 mg/day] based on body surface area) administered during organogenesis and pregnant rabbits at oral lansoprazole doses up to 30 mg/kg/day (16 times the recommended human dose based on body surface area) administered during organogenesis.
A pre- and postnatal developmental toxicity study in rats with additional endpoints to evaluate bone development was performed with lansoprazole at oral doses of 10 to 100 mg/kg/day (0.7 to 6.4 times the maximum recommended human lansoprazole dose of 30 mg based on AUC [area under the plasma concentration-time curve]) administered during organogenesis through lactation. Maternal effects observed at 100 mg/kg/day (6.4 times the maximum recommended human lansoprazole dose of 30 mg based on AUC) included increased gestation period, decreased body weight gain during gestation, and decreased food consumption. The number of stillbirths was increased at this dose, which may have been secondary to maternal toxicity. Body weight of pups was reduced at 100 mg/kg/day starting on postnatal Day 11. Femur weight, femur length, and crown-rump length were reduced at 100 mg/kg/day on postnatal Day 21. Femur weight was still decreased in the 100 mg/kg/day group at age 17 to 18 weeks. Growth plate thickness was decreased in the 100 mg/kg/day males on postnatal Day 21, and was increased in the 30 and 100 mg/kg/day males at age 17 to 18 weeks. The effects on bone parameters were associated with reduction in body weight gain.
8.2 Lactation
Risk Summary
There is no information regarding the presence of lansoprazole in human milk, the effects on the breastfed infant, or the effects on milk production. However, lansoprazole and its metabolites are present in rat milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for lansoprazole delayed-release capsules and any potential adverse effects on the breastfed child from lansoprazole delayed-release capsules or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of lansoprazole have been established in pediatric patients one year to 17 years of age for short-term treatment of symptomatic GERD and erosive esophagitis.
In clinical studies of symptomatic GERD and erosive esophagitis, lansoprazole was not administered beyond 12 weeks in patients one year to 11 years of age. It is not known if lansoprazole is safe and effective if used longer than the recommended duration. Do not exceed the recommended dose and duration of use in pediatric patients (see Juvenile Animal Toxicity Data).
Lansoprazole was not effective in pediatric patients with symptomatic GERD one month to less than one year of age in a multicenter, double-blind, placebo-controlled study. Therefore, safety and effectiveness have not been established in patients less than one year of age. Nonclinical studies in juvenile rats have demonstrated an adverse effect of heart valve thickening and bone changes at lansoprazole doses higher than the maximum recommended equivalent human dose.
Neonate to less than one year of age
The pharmacokinetics of lansoprazole were studied in pediatric patients with GERD aged less than 28 days and one to 11 months. Compared to healthy adults receiving 30 mg, neonates had higher exposure (mean weight-based normalized AUC values 2.04 and 1.88 fold higher at doses of 0.5 and 1 mg/kg/day, respectively). Infants aged ≤10 weeks had clearance and exposure values that were similar to neonates. Infants aged greater than 10 weeks who received 1 mg/kg/day had mean AUC values that were similar to adults who received a 30 mg dose.
Lansoprazole was not found to be effective in a US and Polish four week, multicenter, double-blind, placebo-controlled, parallel-group study of 162 patients between one month and less than 12 months of age with symptomatic GERD based on a medical history of crying/fussing/irritability associated with feedings who had not responded to conservative GERD management (i.e., nonpharmacologic intervention) for seven to 14 days. Patients received lansoprazole as a suspension daily (0.2 to 0.3 mg/kg/day in infants ≤10 weeks of age or 1.0 to 1.5 mg/kg/day in infants greater than 10 weeks or placebo) for up to four weeks of double-blind treatment.
The primary efficacy endpoint was assessed by greater than 50% reduction from baseline in either the percent of feedings with a crying/fussing/irritability episode or the duration (minutes) of a crying/fussing/irritability episode within one hour after feeding.
There was no difference in the percentage of responders between the lansoprazole pediatric suspension group and placebo group (54% in both groups).
There were no adverse events reported in pediatric clinical studies (one month to less than 12 months of age) that were not previously observed in adults.
Based on the results of the Phase 3 efficacy study, lansoprazole was not shown to be effective. Therefore, these results do not support the use of lansoprazole in treating symptomatic GERD in infants.
One year to 11 years of age
In an uncontrolled, open-label, US multicenter study, 66 pediatric patients (one year to 11 years of age) with GERD were assigned, based on body weight, to receive an initial dose of either lansoprazole 15 mg daily if ≤30 kg or lansoprazole 30 mg daily if greater than 30 kg administered for eight to 12 weeks. The lansoprazole dose was increased (up to 30 mg twice daily) in 24 of 66 pediatric patients after two or more weeks of treatment if they remained symptomatic. At baseline, 85% of patients had mild to moderate overall GERD symptoms (assessed by investigator interview), 58% had non-erosive GERD and 42% had erosive esophagitis (assessed by endoscopy).
After eight to 12 weeks of lansoprazole treatment, the intent-to-treat analysis demonstrated an approximate 50% reduction in frequency and severity of GERD symptoms.
Twenty-one of 27 erosive esophagitis patients were healed at eight weeks and 100% of patients were healed at 12 weeks by endoscopy (Table 4).
Table 4. GERD Symptom Improvement and Erosive Esophagitis Healing Rates in Pediatric Patients Age 1 Year to 11 Years of Age GERD
Final Visit* % (n/N)
Symptomatic GERD
Improvement in Overall GERD Symptoms†
76% (47/62‡)
Erosive Esophagitis
Improvement in Overall GERD Symptoms†
81% (22/27)
Healing Rate
100% (27/27)
* At Week 8 or Week 12
† Symptoms assessed by patients diary kept by caregiver.
‡ No data were available for four pediatric patients.
In a study of 66 pediatric patients in the age group one year to 11 years old after treatment with lansoprazole given orally in doses of 15 mg daily to 30 mg twice daily, increases in serum gastrin levels were similar to those observed in adult studies. Median fasting serum gastrin levels increased 89% from 51 pg/mL at baseline to 97 pg/mL [interquartile range (25th to 75th percentile) of 71 to 130 pg/mL] at the final visit.
The pediatric safety of lansoprazole delayed-release capsules has been assessed in 66 pediatric patients aged one to 11 years of age. Of the 66 patients with GERD, 85% (56/66) took lansoprazole for eight weeks and 15% (10/66) took it for 12 weeks.
The most frequently reported (two or more patients) treatment-related adverse reactions in patients one to 11 years of age (N=66) were constipation (5%) and headache (3%).
Twelve years to 17 years of age
In an uncontrolled, open-label, US multicenter study, 87 adolescent patients (12 years to 17 years of age) with symptomatic GERD were treated with lansoprazole for eight to 12 weeks. Baseline upper endoscopies classified these patients into two groups: 64 (74%) non-erosive GERD and 23 (26%) erosive esophagitis (EE). The non-erosive GERD patients received lansoprazole 15 mg daily for eight weeks and the EE patients received lansoprazole 30 mg daily for eight to 12 weeks. At baseline, 89% of these patients had mild to moderate overall GERD symptoms (assessed by investigator interviews). During eight weeks of lansoprazole treatment, adolescent patients experienced a 63% reduction in frequency and a 69% reduction in severity of GERD symptoms based on diary results.
Twenty-one of 22 (95.5%) adolescent erosive esophagitis patients were healed after eight weeks of lansoprazole treatment. One patient remained unhealed after 12 weeks of treatment (Table 5).
Table 5. GERD Symptom Improvement and Erosive Esophagitis Healing Rates in Pediatric Patients Age 12 Years to 17 Years of Age GERD
Final Visit % (n/N)
Symptomatic GERD (All Patients)
Improvement in Overall GERD Symptoms*
73.2% (60/82)†
Non-erosive GERD
Improvement in Overall GERD Symptoms*
71.2% (42/59)†
Erosive Esophagitis
Improvement in Overall GERD Symptoms*
78.3% (18/23)
Healing Rate‡
95.5% (21/22)‡
* Symptoms assessed by patient diary (parents/caregivers as necessary).
† No data available for five patients.
‡ Data from one healed patient was excluded from this analysis due to timing of final endoscopy.
In these 87 adolescent patients, increases in serum gastrin levels were similar to those observed in adult studies, median fasting serum gastrin levels increased 42% from 45 pg/mL at baseline to 64 pg/mL [interquartile range (25th to 75th percentile) of 44 to 88 pg/mL] at the final visit. (Normal serum gastrin levels are 25 to 111 pg/mL.)
The safety of lansoprazole delayed-release capsules has been assessed in these 87 adolescent patients. Of the 87 adolescent patients with GERD, 6% (5/87) took lansoprazole for less than six weeks, 93% (81/87) for six to 10 weeks, and 1% (1/87) for greater than 10 weeks.
The most frequently reported (at least 3%) treatment-related adverse reactions in these patients were headache (7%), abdominal pain (5%), nausea (3%) and dizziness (3%). Treatment-related dizziness, reported in this prescribing information as occurring in less than 1% of adult patients, was reported in this study by three adolescent patients with non-erosive GERD, who had dizziness concurrently with other reactions (such as migraine, dyspnea, and vomiting).
Juvenile Animal Toxicity Data
Heart Valve Thickening
In two oral toxicity studies, thickening of the mitral heart valve occurred in juvenile rats treated with lansoprazole. Heart valve thickening was observed primarily with oral dosing initiated on postnatal Day 7 (age equivalent to neonatal humans) and postnatal Day 14 (human age equivalent of approximately one year) at doses of 250 mg/kg/day and higher (at postnatal Day 7 and postnatal Day 14, respectively 6.2 times and 4.2 times the daily pediatric dose of 15 mg in pediatric patients age one to 11 years weighing 30 kg or less, based on AUC). The treatment durations associated with heart valve thickening ranged from 5 days to 8 weeks. The findings reversed or trended towards reversibility after a 4-week drug-free recovery period. The incidence of heart valve thickening after initiation of dosing on postnatal Day 21 (human age equivalent of approximately two years) was limited to a single rat (1/24) in groups given 500 mg/kg/day for 4 or 8 weeks (approximately 5.2 times the daily pediatric dose of 15 mg in pediatric patients age one to 11 years weighing 30 kg or less, based on AUC). Based on exposure margins, the risk of heart valve injury does not appear to be relevant to patients one year of age and older.
Bone Changes
In an eight-week oral toxicity study in juvenile rats with dosing initiated on postnatal Day 7, doses equal to or greater than 100 mg/kg/day (2.5 times the daily pediatric dose of 15 mg in children age one to 11 years weighing 30 kg or less, based on AUC) produced delayed growth, with impairment of weight gain observed as early as postnatal Day 10 (age equivalent to neonatal humans). At the end of treatment, the signs of impaired growth at 100 mg/kg/day and higher included reductions in body weight (14 to 44% compared to controls), absolute weight of multiple organs, femur weight, femur length, and crown-rump length. Femoral growth plate thickness was reduced only in males and only at the 500 mg/kg/day dose. The effects related to delayed growth persisted through the end of the four-week recovery period. Longer term data were not collected.
8.5 Geriatric Use
Of the total number of patients (n=21,486) in clinical studies of lansoprazole, 16% of patients were aged 65 years and over, while 4% were 75 years and over. No overall differences in safety or effectiveness were observed between these patients and younger patients and other reported clinical experience has not identified significant differences in responses between geriatric and younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3)].
Close8.6 Hepatic Impairment
In patients with various degrees of chronic hepatic impairment the exposure to lansoprazole was increased compared to healthy subjects with normal hepatic function [see Clinical Pharmacology (12.3)]. No dosage adjustment for lansoprazole delayed-release capsules is necessary for patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. The recommended dosage is 15 mg orally daily in patients with severe hepatic impairment (Child-Pugh Class C) [see Dosage and Administration (2.3)].
-
10 OVERDOSAGELansoprazole is not removed from the circulation by hemodialysis. In one reported overdose, a patient consumed 600 mg of lansoprazole with no adverse reaction. Oral lansoprazole doses up to 5000 ...
Lansoprazole is not removed from the circulation by hemodialysis. In one reported overdose, a patient consumed 600 mg of lansoprazole with no adverse reaction. Oral lansoprazole doses up to 5000 mg/kg in rats [approximately 1300 times the 30 mg human dose based on body surface area (BSA)] and in mice (about 675.7 times the 30 mg human dose based on BSA) did not produce deaths or any clinical signs.
In the event of over-exposure, treatment should be symptomatic and supportive.
If over-exposure occurs, call your poison control center at 1-800-222-1222 for current information on the management of poisoning or over-exposure.
Close -
11 DESCRIPTIONThe active ingredient in lansoprazole delayed-release capsules, USP is lansoprazole, USP, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl ...
The active ingredient in lansoprazole delayed-release capsules, USP is lansoprazole, USP, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Lansoprazole, USP has the following structure:
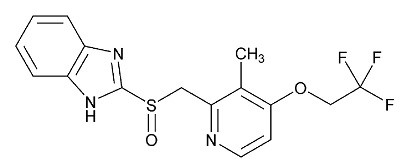
C16H14F3N3O2S M.W. 369.36
Lansoprazole, USP is a white to brownish-white odorless crystalline powder which melts with decomposition at approximately 166°C. Lansoprazole, USP is freely soluble in dimethylformamide; soluble in methanol; sparingly soluble in ethanol; slightly soluble in ethyl acetate, dichloromethane and acetonitrile; very slightly soluble in ether; and practically insoluble in hexane and water.
Lansoprazole, USP is stable when exposed to light for up to two months. The rate of degradation of the compound in aqueous solution increases with decreasing pH. The degradation half-life of the drug substance in aqueous solution at 25°C is approximately 0.5 hour at pH 5 and approximately 18 hours at pH 7.
The lansoprazole delayed-release capsules, USP for oral administration are available in two dosage strengths: 15 mg and 30 mg of lansoprazole, USP per capsule. Each delayed-release capsule contains enteric-coated granules consisting of 15 mg or 30 mg of lansoprazole, USP (active ingredient) and the following inactive ingredients: black iron oxide, gelatin, hypromellose, magnesium carbonate, methacrylic acid copolymer dispersion, propylene glycol, red iron oxide, shellac, sugar spheres (which contain sucrose and corn starch), talc, titanium dioxide, and triethyl citrate. Additionally, 15 mg capsule contains brilliant blue FCF - FD&C blue 1.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lansoprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that suppress gastric acid secretion by specific inhibition of the (H+ ...
12.1 Mechanism of Action
Lansoprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that suppress gastric acid secretion by specific inhibition of the (H+, K+)-ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the parietal cell, lansoprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated gastric acid secretion irrespective of the stimulus. Lansoprazole does not exhibit anticholinergic or histamine type-2 antagonist activity.
12.2 Pharmacodynamics
Antisecretory Activity
After oral administration, lansoprazole was shown to significantly decrease the basal acid output and significantly increase the mean gastric pH and percent of time the gastric pH was greater than three and greater than four. Lansoprazole also significantly reduced meal-stimulated gastric acid output and secretion volume, as well as pentagastrin-stimulated acid output. In patients with hypersecretion of acid, lansoprazole significantly reduced basal and pentagastrin-stimulated gastric acid secretion. Lansoprazole inhibited the normal increases in secretion volume, acidity and acid output induced by insulin.
The intragastric pH results of a five day, pharmacodynamic, crossover study of 15 mg and 30 mg of once daily lansoprazole are presented in Table 6:
Table 6. Mean Antisecretory Effects After Single and Multiple Daily Lansoprazole Dosing Lansoprazole
Parameter
Baseline Value
15 mg
30 mg
Day 1
Day 5
Day 1
Day 5
Mean 24 Hour pH
2.1
2.7*
4.0*
3.6†
4.9†
Mean Nighttime pH
1.9
2.4
3.0*
2.6
3.8†
% Time Gastric pH>3
18
33*
59*
51†
72†
% Time Gastric pH>4
12
22*
49*
41†
66†
NOTE: An intragastric pH of greater than four reflects a reduction in gastric acid by 99%.
* (p<0.05) vs baseline only.
† (p<0.05) vs baseline and lansoprazole 15 mg.
After the initial dose in this study, increased gastric pH was seen within one to two hours with 30 mg of lansoprazole and two to three hours with 15 mg of lansoprazole. After multiple daily dosing, increased gastric pH was seen within the first hour postdosing with 30 mg of lansoprazole and within one to two hours postdosing with 15 mg of lansoprazole.
Acid suppression may enhance the effect of antimicrobials in eradicating Helicobacter pylori (H. pylori). The percentage of time gastric pH was elevated above five and six was evaluated in a crossover study of lansoprazole given daily, twice daily and three times daily (Table 7).
Table 7. Mean Antisecretory Effects After Five Days of Twice Daily and Three Times Daily Dosing Lansoprazole
Parameter
30 mg daily
15 mg twice daily
30 mg twice daily
30 mg three times daily
% Time Gastric pH>5
43
47
59*
77†
% Time Gastric pH>6
20
23
28
45†
* (p<0.05) vs lansoprazole 30 mg daily.
† (p<0.05) vs lansoprazole 30 mg daily, 15 mg and 30 mg twice daily.
The inhibition of gastric acid secretion as measured by intragastric pH gradually returned to normal over two to four days after multiple doses. There was no indication of rebound gastric acidity.
Enterochromaffin-like (ECL) Cell Effects
During lifetime exposure of rats with up to 150 mg/kg/day of lansoprazole dosed seven days per week, marked hypergastrinemia was observed followed by ECL cell proliferation and formation of carcinoid tumors, especially in female rats. Gastric biopsy specimens from the body of the stomach from approximately 150 patients treated continuously with lansoprazole for at least one year did not show evidence of ECL cell effects similar to those seen in rat studies. Longer term data are needed to rule out the possibility of an increased risk of the development of gastric tumors in patients receiving long-term therapy with lansoprazole [see Nonclinical Toxicology (13.1)].
Other Gastric Effects in Humans
Lansoprazole did not significantly affect mucosal blood flow in the fundus of the stomach. Due to the normal physiologic effect caused by the inhibition of gastric acid secretion, a decrease of about 17% in blood flow in the antrum, pylorus, and duodenal bulb was seen. Lansoprazole significantly slowed the gastric emptying of digestible solids. Lansoprazole increased serum pepsinogen levels and decreased pepsin activity under basal conditions and in response to meal stimulation or insulin injection. As with other agents that elevate intragastric pH, increases in gastric pH were associated with increases in nitrate-reducing bacteria and elevation of nitrite concentration in gastric juice in patients with gastric ulcer. No significant increase in nitrosamine concentrations was observed.
Serum Gastrin Effects
In over 2100 patients, median fasting serum gastrin levels increased 50 to 100% from baseline but remained within normal range after treatment with 15 to 60 mg of oral lansoprazole. These elevations reached a plateau within two months of therapy and returned to pretreatment levels within four weeks after discontinuation of therapy.
Increased gastrin causes enterochromaffin-like cell hyperplasia and increased serum CgA levels. The increased CgA levels may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions (5.9)].
Endocrine Effects
Human studies for up to one year have not detected any clinically significant effects on the endocrine system. Hormones studied include testosterone, luteinizing hormone (LH), follicle stimulating hormone (FSH), sex hormone binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEA-S), prolactin, cortisol, estradiol, insulin, aldosterone, parathormone, glucagon, thyroid stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), and somatotropic hormone (STH). Lansoprazole in oral doses of 15 to 60 mg for up to one year had no clinically significant effect on sexual function. In addition, lansoprazole in oral doses of 15 to 60 mg for two to eight weeks had no clinically significant effect on thyroid function. In 24 month carcinogenicity studies in Sprague-Dawley rats with daily lansoprazole dosages up to 150 mg/kg, proliferative changes in the Leydig cells of the testes, including benign neoplasm, were increased compared to control rats.
Other Effects
No systemic effects of lansoprazole on the central nervous system, lymphoid, hematopoietic, renal, hepatic, cardiovascular, or respiratory systems have been found in humans. Among 56 patients who had extensive baseline eye evaluations, no visual toxicity was observed after lansoprazole treatment (up to 180 mg/day) for up to 58 months. After lifetime lansoprazole exposure in rats, focal pancreatic atrophy, diffuse lymphoid hyperplasia in the thymus, and spontaneous retinal atrophy were seen.
12.3 Pharmacokinetics
Absorption:
Lansoprazole delayed-release capsules contain an enteric-coated granule formulation of lansoprazole (because lansoprazole is acid-labile), so that absorption of lansoprazole begins only after the granules leave the stomach. The mean peak plasma levels of lansoprazole occur at approximately 1.7 hours. After a single-dose administration of 15 mg to 60 mg of oral lansoprazole, the peak plasma concentrations (Cmax) of lansoprazole and the area under the plasma concentration curves (AUCs) of lansoprazole were approximately proportional to the administered dose. Lansoprazole does not accumulate and its pharmacokinetics are unaltered by multiple dosing. The absolute bioavailability is over 80%. In healthy subjects, the mean (±SD) plasma half-life was 1.5 (±1.0) hours. Both the Cmax and AUC are diminished by about 50 to 70% if lansoprazole is given 30 minutes after food, compared to the fasting condition. There is no significant food effect if lansoprazole is given before meals.
Distribution:
Lansoprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 0.05 to 5 mcg/mL.
Elimination
Metabolism: Lansoprazole is extensively metabolized in the liver. Two metabolites have been identified in measurable quantities in plasma (the hydroxylated sulfinyl and sulfone derivatives of lansoprazole). These metabolites have very little or no antisecretory activity. Lansoprazole is thought to be transformed into two active species which inhibit acid secretion by blocking the proton pump [(H+, K+)-ATPase enzyme system] at the secretory surface of the gastric parietal cell. The two active species are not present in the systemic circulation. The plasma elimination half-life of lansoprazole is less than two hours while the acid inhibitory effect lasts more than 24 hours. Therefore, the plasma elimination half-life of lansoprazole does not reflect its duration of suppression of gastric acid secretion.
Excretion: Following single-dose oral administration of lansoprazole, virtually no unchanged lansoprazole was excreted in the urine. In one study, after a single oral dose of 14C-lansoprazole, approximately one-third of the administered radiation was excreted in the urine and two-thirds was recovered in the feces. This implies a significant biliary excretion of the lansoprazole metabolites.
One to 17 years of age
The pharmacokinetics of lansoprazole were studied in pediatric patients with GERD aged one to 11 years and 12 to 17 years in two separate clinical studies. In children aged one to 11 years, lansoprazole was dosed 15 mg daily for subjects weighing ≤30 kg and 30 mg daily for subjects weighing greater than 30 kg. Mean Cmax and AUC values observed on Day 5 of dosing were similar between the two dose groups and were not affected by weight or age within each weight-adjusted dose group used in the study. In adolescent subjects aged 12 to 17 years, subjects were randomized to receive lansoprazole at 15 mg or 30 mg daily. Mean Cmax and AUC values of lansoprazole were not affected by body weight or age; and nearly dose-proportional increases in mean Cmax and AUC values were observed between the two dose groups in the study. Overall, lansoprazole pharmacokinetics in pediatric patients aged one to 17 years were similar to those observed in healthy adult subjects.
Geriatric Patients:
The clearance of lansoprazole is decreased in the elderly, with elimination half-life increased approximately 50 to 100%. Because the mean half-life in the elderly remains between 1.9 to 2.9 hours, repeated once daily dosing does not result in accumulation of lansoprazole. Peak plasma levels were not increased in the elderly [see Use in Specific Populations (8.5)].
Male and Female Patients:
In a study comparing 12 male and six female human subjects who received lansoprazole, no sex-related differences were found in pharmacokinetics and intragastric pH results.
Racial or Ethnic Groups:
The pooled mean pharmacokinetic parameters of lansoprazole from twelve US studies (N=513) were compared to the mean pharmacokinetic parameters from two Asian studies (N=20). The mean AUCs of lansoprazole in Asian subjects were approximately twice those seen in pooled US data; however, the inter-individual variability was high. The Cmax values were comparable.
Patients with Renal Impairment:
In patients with severe renal impairment, plasma protein binding decreased by 1 to 1.5% after administration of 60 mg of lansoprazole. Patients with renal impairment had a shortened elimination half-life and decreased total AUC (free and bound). The AUC for free lansoprazole in plasma, however, was not related to the degree of renal impairment; and the Cmax and Tmax (time to reach the maximum concentration) were not different than the Cmax and Tmax from subjects with normal renal function. Therefore, the pharmacokinetics of lansoprazole were not clinically different in patients with mild, moderate or severe renal impairment compared to healthy subjects with normal renal function.
Patients with Hepatic Impairment:
In patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment there was an approximate 3-fold increase in mean AUC compared to healthy subjects with normal hepatic function following multiple oral doses of 30 mg lansoprazole for seven days. The corresponding mean plasma half-life of lansoprazole was prolonged from 1.5 to four hours (Child-Pugh A) or five hours (Child-Pugh B).
In patients with compensated and decompensated cirrhosis, there was an approximate 6- and 5-fold increase in AUC, respectively, compared to healthy subjects with normal hepatic function following a single oral dose of 30 mg lansoprazole [see Dosage and Administration (2.3), Use in Specific Populations (8.6)].
Drug Interaction Studies
Effect of Lansoprazole on Other Drugs
Cytochrome P450 Interactions:
Lansoprazole is metabolized through the cytochrome P450 system, specifically through the CYP3A and CYP2C19 isozymes. Studies have shown that lansoprazole does not have clinically significant interactions with other drugs metabolized by the cytochrome P450 system, such as warfarin, antipyrine, indomethacin, ibuprofen, phenytoin, propranolol, prednisone, diazepam, or clarithromycin in healthy subjects. These compounds are metabolized through various cytochrome P450 isozymes including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A.
Theophylline:
When lansoprazole was administered concomitantly with theophylline (CYP1A2, CYP3A), a minor increase (10%) in the clearance of theophylline was seen. Because of the small magnitude and the direction of the effect on theophylline clearance, this interaction is unlikely to be of clinical concern [see Drug Interactions (7)].
Methotrexate and 7-hydroxymethotrexate:
In an open-label, single-arm, eight day, pharmacokinetic study of 28 adult rheumatoid arthritis patients (who required the chronic use of 7.5 to 15 mg of methotrexate given weekly), administration of seven days of naproxen 500 mg twice daily and lansoprazole 30 mg daily had no effect on the pharmacokinetics of methotrexate and 7-hydroxymethotrexate. While this study was not designed to assess the safety of this combination of drugs, no major adverse reactions were noted. However, this study was conducted with low doses of methotrexate. A drug interaction study with high doses of methotrexate has not been conducted [see Warnings and Precautions (5.10)].
Amoxicillin:
Lansoprazole has also been shown to have no clinically significant interaction with amoxicillin.
Sucralfate:
In a single-dose crossover study examining lansoprazole 30 mg administered alone and concomitantly with sucralfate 1 gram, absorption of lansoprazole was delayed and the bioavailability was reduced by 17% when administered concomitantly with sucralfate [see Dosage and Administration (2.4), Drug Interactions (7)].
Antacids:
In clinical trials, antacids were administered concomitantly with lansoprazole and there was no evidence of a change in the efficacy of lansoprazole.
Clopidogrel:
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. A study of healthy subjects who were CYP2C19 extensive metabolizers, receiving once daily administration of clopidogrel 75 mg alone or concomitantly with lansoprazole 30 mg (n=40), for nine days was conducted. The mean AUC of the active metabolite of clopidogrel was reduced by approximately 14% (mean AUC ratio was 86%, with 90% CI of 80 to 92%) when lansoprazole was coadministered compared to administration of clopidogrel alone.
Pharmacodynamic parameters were also measured and demonstrated that the change in inhibition of platelet aggregation (induced by 5 mcM ADP) was related to the change in the exposure to clopidogrel active metabolite. The effect on exposure to the active metabolite of clopidogrel and on clopidogrel-induced platelet inhibition is not considered clinically important.
Effect of Other Drugs on Lansoprazole
Because lansoprazole is metabolized by CYP2C19 and CYP3A4, inducers and inhibitors of these enzymes may potentially alter exposure of lansoprazole.
Close12.4 Microbiology
Microbiology
Lansoprazole, clarithromycin and/or amoxicillin have been shown to be active against most strains of Helicobacter pylori in vitro and in clinical infections [see Indications and Usage (1.2)].
Helicobacter pylori Pretreatment Resistance
Clarithromycin pretreatment resistance (≥2.0 mcg/mL) was 9.5% (91/960) by E-test and 11.3% (12/106) by agar dilution in the dual and triple therapy clinical trials (M93-125, M93-130, M93-131, M95-392, and M95-399).
Amoxicillin pretreatment susceptible isolates (≤0.25 mcg/mL) occurred in 97.8% (936/957) and 98.0% (98/100) of the patients in the dual and triple therapy clinical trials by E-test and agar dilution, respectively. Twenty-one of 957 patients (2.2%) by E-test, and two of 100 patients (2.0%) by agar dilution, had amoxicillin pretreatment MICs of greater than 0.25 mcg/mL. One patient on the 14 day triple therapy regimen had an unconfirmed pretreatment amoxicillin minimum inhibitory concentration (MIC) of greater than 256 mcg/mL by E-test and the patient was eradicated of H. pylori (Table 8).
Table 8. Clarithromycin Susceptibility Test Results and Clinical/Bacteriological Outcomes* Clarithromycin Pretreatment Results
Clarithromycin Post-treatment Results
H. pylori
negative -
eradicated
H. pylori positive – not
eradicated
Post-treatment susceptibility
results
S†
I†
R†
No MIC
Triple Therapy 14 Day (lansoprazole 30 mg twice daily/amoxicillin 1 g twice daily/clarithromycin 500 mg twice daily) (M95-399, M93-131, M95-392)
Susceptible†
112
105
7
Intermediate†
3
3
Resistant†
17
6
7
4
Triple Therapy 10 Day (lansoprazole 30 mg twice daily/amoxicillin 1 g twice daily/clarithromycin 500 mg twice daily) (M95-399)
Susceptible†
42
40
1
1
Intermediate†
Resistant†
4
1
3
* Includes only patients with pretreatment clarithromycin susceptibility test results
† Susceptible (S) MIC≤0.25 mcg/mL, Intermediate (I) MIC 0.5 to 1.0 mcg/mL, Resistant (R) MIC≥2 mcg/mL
Patients not eradicated of H. pylori following lansoprazole/amoxicillin/clarithromycin triple therapy will likely have clarithromycin resistant H. pylori. Therefore, for those patients who fail therapy, clarithromycin susceptibility testing should be done when possible. Patients with clarithromycin resistant H. pylori should not be treated with lansoprazole/amoxicillin/clarithromycin triple therapy or with regimens which include clarithromycin as the sole antimicrobial agent.
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes: In the dual and triple therapy clinical trials, 82.6% (195/236) of the patients that had pretreatment amoxicillin susceptible MICs (≤0.25 mcg/mL) were eradicated of H. pylori. Of those with pretreatment amoxicillin MICs of greater than 0.25 mcg/mL, three of six had the H. pylori eradicated. A total of 30% (21/70) of the patients failed lansoprazole 30 mg three times daily/amoxicillin 1 g three times daily dual therapy and a total of 12.8% (22/172) of the patients failed the 10 and 14 day triple therapy regimens. Post-treatment susceptibility results were not obtained on 11 of the patients who failed therapy. Nine of the 11 patients with amoxicillin post-treatment MICs that failed the triple therapy regimen also had clarithromycin resistant H. pylori isolates.
Susceptibility Test for Helicobacter pylori: For susceptibility testing information about Helicobacter pylori, see Microbiology section in prescribing information for clarithromycin and amoxicillin.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In two, 24 month carcinogenicity studies, Sprague-Dawley rats were treated with oral lansoprazole doses of 5 to 150 mg/kg/day, about ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In two, 24 month carcinogenicity studies, Sprague-Dawley rats were treated with oral lansoprazole doses of 5 to 150 mg/kg/day, about one to 40 times the exposure on a body surface (mg/m2) basis of a 50 kg person of average height [1.46 m2 body surface area (BSA)] given the recommended human dose of 30 mg/day. Lansoprazole produced dose-related gastric enterochromaffin-like (ECL) cell hyperplasia and ECL cell carcinoids in both male and female rats. It also increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes. In male rats, lansoprazole produced a dose-related increase of testicular interstitial cell adenomas. The incidence of these adenomas in rats receiving doses of 15 to 150 mg/kg/day (four to 40 times the recommended human dose based on BSA) exceeded the low background incidence (range = 1.4 to 10%) for this strain of rat.
In a 24 month carcinogenicity study, CD-1 mice were treated with oral lansoprazole doses of 15 to 600 mg/kg/day, two to 80 times the recommended human dose based on BSA. Lansoprazole produced a dose-related increased incidence of gastric ECL cell hyperplasia. It also produced an increased incidence of liver tumors (hepatocellular adenoma plus carcinoma). The tumor incidences in male mice treated with 300 and 600 mg/kg/day (40 to 80 times the recommended human dose based on BSA) and female mice treated with 150 to 600 mg/kg/day (20 to 80 times the recommended human dose based on BSA) exceeded the ranges of background incidences in historical controls for this strain of mice. Lansoprazole treatment produced adenoma of rete testis in male mice receiving 75 to 600 mg/kg/day (10 to 80 times the recommended human dose based on BSA).
A 26 week p53 (+/-) transgenic mouse carcinogenicity study was not positive.
Lansoprazole was positive in the Ames test and the in vitro human lymphocyte chromosomal aberration assay. Lansoprazole was not genotoxic in the ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) test, the in vivo mouse micronucleus test, or the rat bone marrow cell chromosomal aberration test.
Lansoprazole at oral doses up to 150 mg/kg/day (40 times the recommended human dose based on BSA) was found to have no effect on fertility and reproductive performance of male and female rats.
-
14 CLINICAL STUDIES14.1 Duodenal Ulcer - In a US multicenter, double-blind, placebo-controlled, dose-response (15, 30, and 60 mg of lansoprazole once daily) study of 284 patients with endoscopically documented ...
14.1 Duodenal Ulcer
In a US multicenter, double-blind, placebo-controlled, dose-response (15, 30, and 60 mg of lansoprazole once daily) study of 284 patients with endoscopically documented duodenal ulcer, the percentage of patients healed after two and four weeks was significantly higher with all doses of lansoprazole than with placebo. There was no evidence of a greater or earlier response with the two higher doses compared with lansoprazole 15 mg. Based on this study and the second study described below, the recommended dose of lansoprazole in duodenal ulcer is 15 mg per day (Table 9).
Table 9. Duodenal Ulcer Healing Rates Lansoprazole Week 15 mg daily
(N=68)30 mg daily
(N=74)60 mg daily
(N=70)Placebo
(N=72)2 42.4%* 35.6%* 39.1%* 11.3% 4 89.4%* 91.7%* 89.9%* 46.1% * (p≤0.001) vs placebo Lansoprazole 15 mg was significantly more effective than placebo in relieving day and nighttime abdominal pain and in decreasing the amount of antacid taken per day.
In a second US multicenter study, also double-blind, placebo-controlled, dose-comparison (15 and 30 mg of lansoprazole once daily), and including a comparison with ranitidine, in 280 patients with endoscopically documented duodenal ulcer, the percentage of patients healed after four weeks was significantly higher with both doses of lansoprazole than with placebo. There was no evidence of a greater or earlier response with the higher dose of lansoprazole. Although the 15 mg dose of lansoprazole was superior to ranitidine at four weeks, the lack of significant difference at two weeks and the absence of a difference between 30 mg of lansoprazole and ranitidine leaves the comparative effectiveness of the two agents undetermined (Table 10) [see Indications and Usage (1.1)].
Table 10. Duodenal Ulcer Healing Rates Lansoprazole Ranitidine Week
15 mg daily
(N=80)30 mg daily
(N=77)300 mg h.s.
(N=82)Placebo
(N=41)2 35.0% 44.2% 30.5% 34.2% 4 92.3%* 80.3%† 70.5%† 47.5% * (p≤0.05) vs placebo and ranitidine.
† (p≤0.05) vs placebo.
14.2 Eradication of H. pylori to Reduce the Risk of Duodenal Ulcer Recurrence
Randomized, double-blind clinical studies performed in the US in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an ulcer within one year) evaluated the efficacy of lansoprazole in combination with amoxicillin and clarithromycin as triple 14 day therapy or in combination with amoxicillin as dual 14 day therapy for the eradication of H. pylori. Based on the results of these studies, the safety and efficacy of two different eradication regimens were established:
Triple therapy: Lansoprazole 30 mg twice daily/amoxicillin 1 g twice daily/clarithromycin 500 mg twice daily
Dual therapy: Lansoprazole 30 mg three times daily/amoxicillin 1 g three times dailyAll treatments were for 14 days. H. pylori eradication was defined as two negative tests (culture and histology) at four to six weeks following the end of treatment.
Triple therapy was shown to be more effective than all possible dual therapy combinations. Dual therapy was shown to be more effective than both monotherapies. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence.
A randomized, double-blind clinical study performed in the US in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an ulcer within one year) compared the efficacy of lansoprazole triple therapy for 10 and 14 days. This study established that the 10 day triple therapy was equivalent to the 14 day triple therapy in eradicating H. pylori (Tables 11 and 12) [see Indications and Usage (1.2)].
Table 11. H. pylori Eradication Rates – Triple Therapy (Lansoprazole/amoxicillin/clarithromycin)
Percent of Patients Cured
[95% Confidence Interval]
(Number of patients)
Study
Duration
Triple Therapy
Evaluable
Analysis*
Triple Therapy
Intent-to-Treat Analysis†M93-131
14 days
92‡
[80.0 to 97.7](N=48)
86‡
[73.3 to 93.5]
(N=55)M95-392
14 days
86§
[75.7 to 93.6]
(N=66)83§
[72.0 to 90.8]
(N=70)
M95-399¶
14 days
85
[77.0 to 91.0]
(N=113)
82
[73.9 to 88.1]
(N=126)
10 days
84
[76.0 to 89.8]
(N=123)
81
[73.9 to 87.6]
(N=135)
* Based on evaluable patients with confirmed duodenal ulcer (active or within one year) and H. pylori infection at baseline defined as at least two of three positive endoscopic tests from CLOtest, histology and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the evaluable analysis as failures of therapy.
† Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within one year). All dropouts were included as failures of therapy.
‡ (p<0.05) vs lansoprazole/amoxicillin and lansoprazole/clarithromycin dual therapy.
§ (p<0.05) vs clarithromycin/amoxicillin dual therapy.
¶ The 95% confidence interval for the difference in eradication rates, 10 day minus 14 day is (-10.5, 8.1) in the evaluable analysis and (-9.7, 9.1) in the intent-to-treat analysis.
Table 12. H. pylori Eradication Rates – 14 Day Dual Therapy (Lansoprazole/amoxicillin)
Percent of Patients Cured
[95% Confidence Interval]
(Number of patients)
Study
Dual Therapy
EvaluableAnalysis*
Dual Therapy
Intent-to-Treat Analysis†M93-131
77‡
[62.5 to 87.2]
(N=51)70‡
[56.8 to 81.2]
(N=60)M93-125
66§
[51.9 to 77.5](N=58)
61§
[48.5 to 72.9](N=67)
* Based on evaluable patients with confirmed duodenal ulcer (active or within one year) and H. pylori infection at baseline defined as at least two of three positive endoscopic tests from CLOtest, histology and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy.
† Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within one year). All dropouts were included as failures of therapy.
‡ (p<0.05) vs lansoprazole alone.
§ (p<0.05) vs lansoprazole alone or amoxicillin alone.
14.3 Maintenance of Healed Duodenal Ulcers
Lansoprazole has been shown to prevent the recurrence of duodenal ulcers. Two independent, double-blind, multicenter, controlled trials were conducted in patients with endoscopically confirmed healed duodenal ulcers. Patients remained healed significantly longer and the number of recurrences of duodenal ulcers was significantly less in patients treated with lansoprazole than in patients treated with placebo over a 12 month period (Table 13) [see Indications and Usage (1.3)].
Table 13. Endoscopic Remission Rates Trial
Drug
No. of Pts.Percent in Endoscopic Remission
0 to 3 mo.
0 to 6 mo.
0 to 12 mo.
#1
Lansoprazole 15 mg daily
86
90%*
87%*
84%*
Placebo
83
49%
41%
39%
#2
Lansoprazole 30 mg daily
18
94%*
94%*
85%*
Lansoprazole 15 mg daily
15
87%*
79%*
70%*
Placebo
15
33%
0%
0%
%=Life Table Estimate
* (p≤0.001) vs placebo.
In trial #2, no significant difference was noted between lansoprazole 15 mg and 30 mg in maintaining remission.
14.4 Gastric Ulcer
In a US multicenter, double-blind, placebo-controlled study of 253 patients with endoscopically documented gastric ulcer, the percentage of patients healed at four and eight weeks was significantly higher with lansoprazole 15 mg and 30 mg once a day than with placebo (Table 14) [see Indications and Usage (1.4)].
Table 14. Gastric Ulcer Healing Rates Week
Lansoprazole
Placebo(N=64)
15 mg daily
(N=65)
30 mg daily
(N=63)
60 mg daily
(N=61)
4
64.6%*
58.1%*
53.3%*
37.5%
8
92.2%*
96.8%*
93.2%*
76.7%
* (p≤0.05) vs placebo.
Patients treated with any lansoprazole dose reported significantly less day and night abdominal pain along with fewer days of antacid use and fewer antacid tablets used per day than the placebo group.
Independent substantiation of the effectiveness of lansoprazole 30 mg was provided by a meta-analysis of published and unpublished data.
14.5 Healing of NSAID-Associated Gastric Ulcer
In two US and Canadian multicenter, double-blind, active-controlled studies in patients with endoscopically confirmed NSAID-associated gastric ulcer who continued their NSAID use, the percentage of patients healed after eight weeks was statistically significantly higher with 30 mg of lansoprazole than with the active control. A total of 711 patients were enrolled in the study, and 701 patients were treated. Patients ranged in age from 18 to 88 years (median age 59 years), with 67% female patients and 33% male patients. Race was distributed as follows: 87% Caucasian, 8% Black, 5% Other. There was no statistically significant difference between lansoprazole 30 mg daily and the active control on symptom relief (i.e., abdominal pain) (Table 15) [see Indications and Usage (1.5)].
Table 15. NSAID-Associated Gastric Ulcer Healing Rates* Study #1
Lansoprazole
30 mg daily
Active Control†
Week 4
60% (53/88)‡
28% (23/83)
Week 8
79% (62/79)‡
55% (41/74)
Study #2
Lansoprazole
30 mg daily
Active Control†
Week 4
53% (40/75)
38% (31/82)
Week 8
77% (47/61)‡
50% (33/66)
* Actual observed ulcer(s) healed at time points ±2 days.
† Dose for healing of gastric ulcer.
‡ (p≤0.05) vs the active control.
14.6 Risk Reduction of NSAID-Associated Gastric Ulcer
In one large US, multicenter, double-blind, placebo- and misoprostol-controlled (misoprostol blinded only to the endoscopist) study in patients who required chronic use of an NSAID and who had a history of an endoscopically documented gastric ulcer, the proportion of patients remaining free from gastric ulcer at four, eight, and 12 weeks was significantly higher with 15 or 30 mg of lansoprazole than placebo. A total of 537 patients were enrolled in the study, and 535 patients were treated. Patients ranged in age from 23 to 89 years (median age 60 years), with 65% female patients and 35% male patients. Race was distributed as follows: 90% Caucasian, 6% Black, 4% Other. The 30 mg dose of lansoprazole demonstrated no additional benefit in risk reduction of the NSAID-associated gastric ulcer than the 15 mg dose (Table 16) [see Indications and Usage (1.6)].
Table 16. Proportion of Patients Remaining Free of Gastric Ulcers* Week
Lansoprazole
15 mg daily
Lansoprazole
30 mg daily
Misoprostol
200 mcg four times daily
Placebo
(N=121)
(N=116)
(N=106)
(N=112)
4
90%
92%
96%
66%
8
86%
88%
95%
60%
12
80%
82%
93%
51%
* % = Life Table Estimate
(p<0.001) Lansoprazole 15 mg daily vs placebo; lansoprazole 30 mg daily vs placebo; and misoprostol 200 mcg four times daily vs placebo.
(p<0.05) Misoprostol 200 mcg four times daily vs lansoprazole 15 mg daily; and misoprostol 200 mcg four times daily vs lansoprazole 30 mg daily.
14.7 Symptomatic Gastroesophageal Reflux Disease (GERD)
Symptomatic GERD: In a US, multicenter, double-blind, placebo-controlled study of 214 patients with frequent GERD symptoms, but no esophageal erosions by endoscopy, significantly greater relief of heartburn associated with GERD was observed with the administration of lansoprazole 15 mg once daily up to eight weeks than with placebo. No significant additional benefit from lansoprazole 30 mg once daily was observed.
The intent-to-treat analyses demonstrated significant reduction in frequency and severity of day and night heartburn. Data for frequency and severity for the eight week treatment period are presented in Table 17 and in Figures 1 and 2:
Table 17. Frequency of Heartburn
VariablePlacebo
(n=43)
Lansoprazole
15 mg
(n=80)
Lansoprazole
30 mg
(n=86)
Median
% of Days without Heartburn
Week 1
0%
71%*
46%*
Week 4
11%
81%*
76%*
Week 8
13%
84%*
82%*
% of Nights without Heartburn
Week 1
17%
86%*
57%*
Week 4
25%
89%*
73%*
Week 8
36%
92%*
80%*
* (p<0.01) vs placebo.
Figure 1
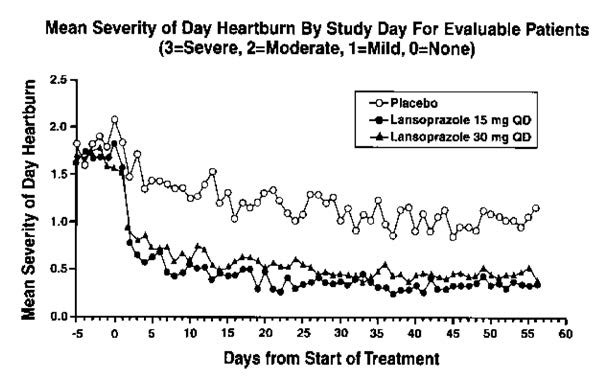
Figure 2
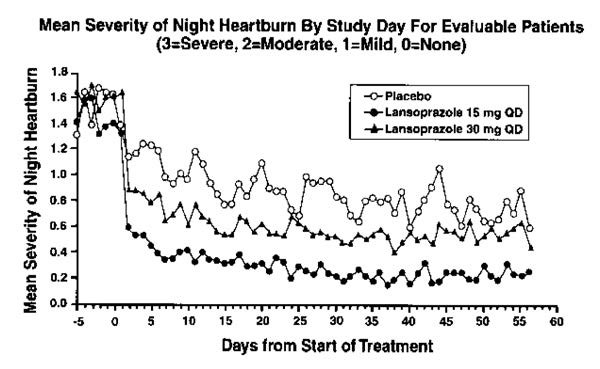
In two US, multicenter double-blind, ranitidine-controlled studies of 925 total patients with frequent GERD symptoms, but no esophageal erosions by endoscopy, lansoprazole 15 mg was superior to ranitidine 150 mg (twice daily) in decreasing the frequency and severity of day and night heartburn associated with GERD for the eight week treatment period. No significant additional benefit from lansoprazole 30 mg once daily was observed [see Indications and Usage (1.7)].
14.8 Erosive Esophagitis
In a US, multicenter, double-blind, placebo-controlled study of 269 patients entering with an endoscopic diagnosis of esophagitis with mucosal grading of two or more and grades three and four signifying erosive disease, the percentages of patients with healing are presented in Table 18:
Table 18. Erosive Esophagitis Healing Rates Week
Lansoprazole
Placebo(N=63)
15 mg daily
(N=69)
30 mg daily
(N=65)
60 mg daily
(N=72)
4
67.6%*
81.3%*,†
80.6%*,†
32.8%
6
87.7%*
95.4%*
94.3%*
52.5%
8
90.9%*
95.4%*
94.4%*
52.5%
* (p≤0.001) vs placebo.
† (p≤0.05) vs lansoprazole 15 mg.
In this study, all lansoprazole groups reported significantly greater relief of heartburn and less day and night abdominal pain along with fewer days of antacid use and fewer antacid tablets taken per day than the placebo group. Although all doses were effective, the earlier healing in the higher two doses suggests 30 mg daily as the recommended dose.
Lansoprazole was also compared in a US multicenter, double-blind study to a low dose of ranitidine in 242 patients with erosive reflux esophagitis. Lansoprazole at a dose of 30 mg was significantly more effective than ranitidine 150 mg twice daily as shown below (Table 19).
Table 19. Erosive Esophagitis Healing Rates Week
Lansoprazole
30 mg daily
(N=115)
Ranitidine
150 mg twice daily
(N=127)
2
66.7%*
38.7%
4
82.5%*
52.0%
6
93.0%*
67.8%
8
92.1%*
69.9%
* (p≤0.001) vs ranitidine.
In addition, patients treated with lansoprazole reported less day and nighttime heartburn and took less antacid tablets for fewer days than patients taking ranitidine 150 mg twice daily.
Although this study demonstrates effectiveness of lansoprazole in healing erosive esophagitis, it does not represent an adequate comparison with ranitidine because the recommended ranitidine dose for esophagitis is 150 mg four times daily, twice the dose used in this study.
In the two trials described and in several smaller studies involving patients with moderate to severe erosive esophagitis, lansoprazole produced healing rates similar to those shown above.
In a US, multicenter, double-blind, active-controlled study, 30 mg of lansoprazole was compared with ranitidine 150 mg twice daily in 151 patients with erosive reflux esophagitis that was poorly responsive to a minimum of 12 weeks of treatment with at least one H2-receptor antagonist given at the dose indicated for symptom relief or greater, namely, cimetidine 800 mg/day, ranitidine 300 mg/day, famotidine 40 mg/day or nizatidine 300 mg/day. Lansoprazole 30 mg was more effective than ranitidine 150 mg twice daily in healing reflux esophagitis, and the percentage of patients with healing were as follows. This study does not constitute a comparison of the effectiveness of histamine H2-receptor antagonists with lansoprazole, as all patients had demonstrated unresponsiveness to the histamine H2-receptor antagonist mode of treatment. It does indicate, however, that lansoprazole may be useful in patients failing on a histamine H2-receptor antagonist (Table 20) [see Indications and Usage (1.7)].
Table 20. Reflux Esophagitis Healing Rates in Patients Poorly Responsive to Histamine H2-Receptor Antagonist Therapy Week
Lansoprazole
30 mg daily
(N=100)
Ranitidine
150 mg twice daily
(N=51)
4
74.7%*
42.6%
8
83.7%*
32.0%
* (p≤0.001) vs ranitidine.
14.9 Maintenance of Healing of Erosive Esophagitis
Two independent, double-blind, multicenter, controlled trials were conducted in patients with endoscopically confirmed healed esophagitis. Patients remained in remission significantly longer and the number of recurrences of erosive esophagitis was significantly less in patients treated with lansoprazole than in patients treated with placebo over a 12 month period (Table 21).
Table 21. Endoscopic Remission Rates Percent in Endoscopic Remission
Trial
Drug
No. of Pts.
0 to 3 mo.
0 to 6 mo.
0 to 12 mo.
#1
Lansoprazole 15 mg daily
59
83%*
81%*
79%*
Lansoprazole 30 mg daily
56
93%*
93%*
90%*
Placebo
55
31%
27%
24%
#2
Lansoprazole 15 mg daily
50
74%*
72%*
67%*
Lansoprazole 30 mg daily
49
75%*
72%*
55%*
Placebo
47
16%
13%
13%
%=Life Table Estimate
* (p≤0.001) vs placebo.
Regardless of initial grade of erosive esophagitis, lansoprazole 15 and 30 mg were similar in maintaining remission.
In a US, randomized, double-blind study, lansoprazole 15 mg daily (n=100) was compared with ranitidine 150 mg twice daily (n=106), at the recommended dosage, in patients with endoscopically-proven healed erosive esophagitis over a 12 month period. Treatment with lansoprazole resulted in patients remaining healed (Grade 0 lesions) of erosive esophagitis for significantly longer periods of time than those treated with ranitidine (p<0.001). In addition, lansoprazole was significantly more effective than ranitidine in providing complete relief of both daytime and nighttime heartburn. Patients treated with lansoprazole remained asymptomatic for a significantly longer period of time than patients treated with ranitidine [see Indications and Usage (1.9)].
Close14.10 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
In open studies of 57 patients with pathological hypersecretory conditions, such as Zollinger-Ellison syndrome (ZES) with or without multiple endocrine adenomas, lansoprazole significantly inhibited gastric acid secretion and controlled associated symptoms of diarrhea, anorexia and pain. Doses ranging from 15 mg every other day to 180 mg per day maintained basal acid secretion below 10 mEq/hr in patients without prior gastric surgery and below 5 mEq/hr in patients with prior gastric surgery.
Initial doses were titrated to the individual patient need, and adjustments were necessary with time in some patients [see Dosage and Administration (2.1)]. Lansoprazole was well-tolerated at these high-dose levels for prolonged periods (greater than four years in some patients). In most ZES patients, serum gastrin levels were not modified by lansoprazole. However, in some patients, serum gastrin increased to levels greater than those present prior to initiation of lansoprazole therapy [see Indications and Usage (1.10)].
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLansoprazole Delayed-Release Capsules, USP are available as follows: 15 mg - hard gelatin capsules, with a light-blue opaque cap and flesh-colored opaque body, imprinted with “93” and “7350” ...
Lansoprazole Delayed-Release Capsules, USP are available as follows:
15 mg - hard gelatin capsules, with a light-blue opaque cap and flesh-colored opaque body, imprinted with “93” and “7350”, filled with off-white to beige pellets, in bottles of 30 (NDC 0093-7350-56).
30 mg - hard gelatin capsules, with a light-gray opaque cap and flesh-colored opaque body, imprinted with “93” and “7351”, filled with off-white to beige pellets, in bottles of 30 (NDC 0093-7351-56).
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Advise patients to: Acute Tubulointerstitial Nephritis - To call their healthcare provider ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Advise patients to:
Acute Tubulointerstitial Nephritis
To call their healthcare provider if they experience signs and/or symptoms associated with acute tubulointerstitial nephritis [see Warnings and Precautions (5.2)].
Clostridium difficile-Associated Diarrhea
To immediately call their healthcare provider if they experience diarrhea that does not improve [see Warnings and Precautions (5.3)].
Bone Fracture
To report any fractures, especially of the hip, wrist or spine, to their healthcare provider [see Warnings and Precautions (5.4)].
Severe Cutaneous Adverse Reactions
To discontinue lansoprazole delayed-release capsules and immediately call their healthcare provider for further evaluation [see Warnings and Precautions (5.5)].
Cutaneous and Systemic Lupus Erythematosus
To immediately call their healthcare provider for any new or worsening of symptoms associated with cutaneous or systemic lupus erythematosus [see Warnings and Precautions (5.6)].
Cyanocobalamin (Vitamin B12) Deficiency
To report any clinical symptoms that may be associated with cyanocobalamin deficiency to their healthcare provider, if they have been receiving lansoprazole for longer than three years [see Warnings and Precautions (5.7)].
Hypomagnesemia and Mineral Metabolism
To report any clinical symptoms that may be associated with hypomagnesemia, hypocalcemia, and/or hypokalemia to their healthcare provider, if they have been receiving lansoprazole for at least three months [see Warnings and Precautions (5.8)].
Drug Interactions
Advise patients to report to their healthcare provider if they are taking rilpivirine-containing products [see Contraindications (4)] or high-dose methotrexate [see Warnings and Precautions (5.10)].
Pregnancy
Advise a pregnant woman of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Administration
- Missed doses: If a dose is missed, administer as soon as possible. However, if the next scheduled dose is due, do not take the missed dose, and take the next dose on time. Do not take two doses at one time to make up for a missed dose.
- Lansoprazole delayed-release capsules should be taken before eating.
- Do not crush or chew lansoprazole delayed-release capsules.
- Take lansoprazole delayed-release capsules at least 30 minutes prior to sucralfate.
Lansoprazole Delayed-Release Capsules
- Swallow whole; do not chew.
- For patients who have difficulty swallowing capsules:
- Lansoprazole delayed-release capsules can be opened and sprinkled on applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
- Lansoprazole delayed-release capsules may also be emptied into a small volume of either apple juice, orange juice or tomato juice
- Alternatively, lansoprazole delayed-release capsules can be administered with apple juice via nasogastric tube
- See the Instructions for Use for a description of all preparation and administration instructions
Dispense with Medication Guide available at: www.tevausa.com/medguides
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Kfar Saba, 4410202, IsraelManufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Rev. R 3/2022
Close -
MEDICATION GUIDEDispense with Medication Guide available at: www.tevausa.com/medguides - MEDICATION GUIDE - Lansoprazole (lan soe’ pra zole) Delayed-Release Capsules, for oral use - What is the most ...
Dispense with Medication Guide available at: www.tevausa.com/medguides
MEDICATION GUIDE
Lansoprazole (lan soe’ pra zole) Delayed-Release Capsules, for oral use
What is the most important information that I should know about lansoprazole delayed-release capsules?
You should take lansoprazole delayed-release capsules exactly as prescribed, at the lowest dose possible and for the shortest time needed.
Lansoprazole delayed-release capsules may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your doctor.
Lansoprazole delayed-release capsules can cause serious side effects, including:
- A type of kidney problem (acute tubulointerstitial nephritis). Some people who take proton pump inhibitor (PPI) medicines, including lansoprazole delayed-release capsules, may develop a kidney problem called acute tubulointerstitial nephritis that can happen at any time during treatment with PPI medicines including lansoprazole delayed-release capsules. Call your doctor right away if you have a decrease in the amount that you urinate or if you have blood in your urine.
- Diarrhea caused by an infection (Clostridium difficile) in your intestines. Call your doctor right away if you have watery stools or stomach pain that does not go away. You may or may not have a fever.
- Bone fractures (hip, wrist, or spine). Bone fractures in the hip, wrist, or spine may happen in people who take multiple daily doses of PPI medicines and for a long period of time (a year or longer). Tell your doctor if you have a bone fracture, especially in the hip, wrist, or spine.
- Certain types of lupus erythematosus. Lupus erythematosus is an autoimmune disorder (the body’s immune cells attack other cells or organs in the body). Some people who take PPI medicines, including lansoprazole delayed-release capsules, may develop certain types of lupus erythematosus or have worsening of the lupus they already have. Call your doctor right away if you have new or worsening joint pain or a rash on your cheeks or arms that gets worse in the sun.
Talk to your doctor about your risk of these serious side effects.
Lansoprazole delayed-release capsules can have other serious side effects. See “What are the possible side effects of lansoprazole delayed-release capsules?”.
What are lansoprazole delayed-release capsules?
A prescription medicine called a proton pump inhibitor (PPI) used to reduce the amount of acid in your stomach.
In adults, lansoprazole delayed-release capsules are used for:
- 4 weeks for the healing and symptom relief of duodenal ulcers.
- 10 to 14 days with certain antibiotics to treat an infection caused by bacteria called H. pylori.
- maintaining healing of duodenal ulcers. Lansoprazole delayed-release capsules have not been studied beyond 12 months for this purpose.
- up to 8 weeks for the healing and symptom relief of stomach ulcers.
- up to 8 weeks for the healing of stomach ulcers in people taking pain medicines called nonsteroidal anti-inflammatory drugs (NSAIDs). Lansoprazole delayed-release capsules have not been studied beyond 8 weeks for this purpose.
- reducing the risk of stomach ulcers in people who are at risk of developing stomach ulcers with NSAIDs. Lansoprazole delayed-release capsules have not been studied beyond 12 weeks for this purpose.
- up to 8 weeks to treat heartburn and other symptoms that happen with gastroesophageal reflux disease (GERD). GERD happens when acid in your stomach backs up into the tube (esophagus) that connects your mouth to your stomach. This may cause a burning feeling in your chest or throat, sour taste or burping.
- up to 8 weeks for the healing and symptom relief of acid-related damage to the lining of the esophagus (called erosive esophagitis or EE). Your doctor may prescribe another 8 to 16 weeks of lansoprazole delayed-release capsules for patients whose EE does not improve or whose symptoms return.
- maintaining healing of EE. Lansoprazole delayed-release capsules have not been studied beyond 12 months for this purpose.
- the long-term treatment of conditions where your stomach makes too much acid. This includes a rare condition called Zollinger-Ellison syndrome.
Children:
Give lansoprazole delayed-release capsules exactly as prescribed by your child’s doctor. Do not increase the dose of lansoprazole delayed-release capsules or give your child lansoprazole delayed-release capsules longer than the amount of time your doctor tells you to.
In children 1 to 11 years of age, lansoprazole delayed-release capsules are used for:
- up to 12 weeks to treat heartburn and other symptoms that can happen with GERD.
- up to 12 weeks for the healing and symptom relief of EE.
In children 12 to 17 years of age, lansoprazole delayed-release capsules are used for:
- up to 8 weeks to treat heartburn and other symptoms that can happen with GERD.
- up to 8 weeks for the healing and symptom relief of EE.
Lansoprazole delayed-release capsules are not recommended for treating the symptoms of GERD in children less than 1 year of age and may harm them.
Do not take lansoprazole delayed-release capsules if you are:
- allergic to lansoprazole, any other PPI medicine, or any of the ingredients in lansoprazole delayed-release capsules. See the end of this Medication Guide for a complete list of ingredients in lansoprazole delayed-release capsules.
- taking a medicine that contains rilpivirine (EDURANT®, COMPLERA®, ODEFSEY®, JULUCA®) used to treat HIV-1 (Human Immunodeficiency Virus).
Before you take lansoprazole delayed-release capsules, tell your doctor about all of your medical conditions, including if you:
- have low magnesium, calcium, potassium or sodium levels in your blood or you are taking a diuretic.
- have liver problems.
- are pregnant, think you may be pregnant or plan to become pregnant. Lansoprazole delayed-release capsules may harm your unborn baby. Talk to your doctor about the possible risks to an unborn baby if lansoprazole delayed-release capsules is taken during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if lansoprazole passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take lansoprazole delayed-release capsules.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your doctor if you take methotrexate (OTREXUP®, RASUVO®, TREXALL®, REDITREX®, XATMEP®).
How should I take lansoprazole delayed-release capsules?
- Take lansoprazole delayed-release capsules exactly as prescribed by your doctor.
- Do not change your dose or stop taking lansoprazole delayed-release capsules without talking to your doctor.
- Take lansoprazole delayed-release capsules before meals.
Lansoprazole delayed-release capsules:
- Swallow lansoprazole delayed-release capsules whole.
- Do not crush or chew lansoprazole delayed-release capsules.
- If you have trouble swallowing a whole capsule, you can open the capsule and take the contents with certain foods or juices. See the “Instructions for Use” at the end of this Medication Guide for instructions on how to take lansoprazole delayed-release capsules with certain foods or juices.
- See the “Instructions for Use” at the end of this Medication Guide for instructions on how to mix and give lansoprazole delayed-release capsules through a nasogastric tube (NG tube).
- If you miss a dose of lansoprazole delayed-release capsules, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose. Take your next dose at your regular time. Do not take 2 doses at the same time.
- If you take too many lansoprazole delayed-release capsules, call your doctor or your poison control center at 1-800-222-1222 right away or go to the nearest hospital emergency room.
What are the possible side effects of lansoprazole delayed-release capsules?
Lansoprazole delayed-release capsules can cause serious side effects, including:
- See “What is the most important information that I should know about lansoprazole delayed-release capsules?”.
- Low vitamin B12 levels in the body can happen in people who have taken lansoprazole for a long time (more than 3 years). Tell your doctor if you have symptoms of low vitamin B12 levels, including shortness of breath, lightheadedness, irregular heartbeat, muscle weakness, pale skin, feeling tired, mood changes, and tingling or numbness in the arms and legs.
- Stomach growths (fundic gland polyps). People who take PPI medicines for a long time have an increased risk of developing a certain type of stomach growth called fundic gland polyps, especially after taking PPI medicines for more than 1 year.
- Low magnesium levels in the body can happen in people who have taken lansoprazole for at least 3 months. Tell your doctor right away if you have symptoms of low magnesium levels, including seizures, dizziness, irregular heartbeat, jitteriness, muscle aches or weakness, and spasms of hands, feet or voice.
- Severe skin reactions. Lansoprazole delayed-release capsules can cause rare but severe skin reactions that may affect any part of your body. These serious skin reactions may need to be treated in a hospital and may be life threatening:
- Skin rash which may have blistering, peeling or bleeding on any part of your skin (including your lips, eyes,
mouth, nose, genitals, hands or feet).
- You may also have fever, chills, body aches, shortness of breath, or enlarged lymph nodes.
Stop taking lansoprazole delayed-release capsules and call your doctor right away. These symptoms may be the first sign of a severe skin reaction.
The most common side effects of lansoprazole delayed-release capsules include: diarrhea, stomach-area (abdomen) pain, nausea and constipation.
These are not all the possible side effects of lansoprazole delayed-release capsules.
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
How should I store lansoprazole delayed-release capsules?
Store lansoprazole delayed-release capsules between 68°F to 77°F (20°C to 25°C).
Keep lansoprazole delayed-release capsules and all medicines out of the reach of children.
General information about the safe and effective use of lansoprazole delayed-release capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use lansoprazole delayed-release capsules for conditions for which they were not prescribed. Do not give lansoprazole delayed-release capsules to other people, even if they have the same symptoms that you have. They may harm them. You can ask your doctor or pharmacist for information about lansoprazole delayed-release capsules that is written for health professionals.
What are the ingredients in lansoprazole delayed-release capsules?
Active ingredient: lansoprazole.
Inactive ingredients: black iron oxide, gelatin, hypromellose, magnesium carbonate, methacrylic acid copolymer dispersion, propylene glycol, red iron oxide, shellac, sugar spheres (which contain sucrose and corn starch), talc, titanium dioxide, and triethyl citrate. Additionally, 15 mg capsule contains brilliant blue FCF - FD&C blue 1.
Brands listed are the trademarks of their respective owners.
Manufactured In Israel By: Teva Pharmaceutical Ind. Ltd., Kfar Saba, 4410202, Israel
Manufactured For: Teva Pharmaceuticals, Parsippany, NJ 07054
For more information, call 1-888-838-2872.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Rev. H 3/2022
CloseINSTRUCTIONS FOR USE
Lansoprazole (lan soe’ pra zole) Delayed-Release Capsules, for oral use
Important:
- Take lansoprazole delayed-release capsules before meals.
- Do not crush or chew lansoprazole delayed-release capsules.
- Lansoprazole delayed-release capsules should only be used with the foods and juices listed below.
Lansoprazole delayed-release capsules
Taking lansoprazole delayed-release capsules with certain foods:
You can only use applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
- Open the capsule.
- Sprinkle the granules on 1 tablespoon of applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
- Swallow right away.
Taking lansoprazole delayed-release capsules with certain juices:
You can only use apple juice, orange juice or tomato juice.
- Open the capsule.
- Sprinkle the granules into 60 mL (about ¼ cup) of apple juice, orange juice or tomato juice.
- Stir.
- Swallow right away.
- To make sure that the entire dose is taken, add 1/2 cup or more of juice to the glass, stir and swallow right away.
Giving lansoprazole delayed-release capsules through a nasogastric tube (NG tube) size 16 French or larger:
You can only use apple juice.
- Place 40 mL of apple juice into a clean container.
- Open the capsule and empty the granules into the container of apple juice.
- Use a catheter-tip syringe to draw up the apple juice and granule mixture.
- Gently mix the catheter-tip syringe to keep the granules from settling.
- Attach the catheter-tip syringe to the NG tube.
- Give the mixture right away through the NG tube that goes into the stomach. Do not save the apple juice and granule mixture for later use.
- Refill the catheter-tip syringe with 40 mL of apple juice and mix gently. Flush the NG tube with apple juice.
How should I store lansoprazole delayed-release capsules?
- Store lansoprazole delayed-release capsules at room temperature between 68°F to 77°F (20°C to 25°C).
Keep lansoprazole delayed-release capsules and all medicines out of the reach of children.
This Instruction for Use has been approved by the U.S. Food and Drug Administration.
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Kfar Saba, 4410202, IsraelManufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Rev. F 1/2022
-
Package/Label Display PanelNDC 0093-7350-56 - Lansoprazole Delayed-Release Capsules, USP - 15 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 30 Capsules - image 1
NDC 0093-7350-56
Lansoprazole Delayed-Release Capsules, USP
15 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules

image 1
Close -
Package/Label Display PanelNDC 0093-7351-56 - Lansoprazole Delayed-Release Capsules, USP - 30 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 30 Capsules - image 2
NDC 0093-7351-56
Lansoprazole Delayed-Release Capsules, USP
30 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules

image 2
Close -
INGREDIENTS AND APPEARANCEProduct Information
LANSOPRAZOLE lansoprazole capsule, delayed release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0093-7350 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANSOPRAZOLE (UNII: 0K5C5T2QPG) (LANSOPRAZOLE - UNII:0K5C5T2QPG) LANSOPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM CARBONATE (UNII: 0E53J927NA) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color blue (light-blue) , white (flesh-colored) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 93;7350;93;7350 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0093-7350-56 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077255 11/11/2009 LANSOPRAZOLE lansoprazole capsule, delayed release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0093-7351 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANSOPRAZOLE (UNII: 0K5C5T2QPG) (LANSOPRAZOLE - UNII:0K5C5T2QPG) LANSOPRAZOLE 30 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM CARBONATE (UNII: 0E53J927NA) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color gray (light-gray) , white (flesh-colored) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 93;7351;93;7351 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0093-7351-56 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077255 11/11/2009
CloseLabeler - Teva Pharmaceuticals USA, Inc. (001627975)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LANSOPRAZOLE capsule, delayed release
Number of versions: 24
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jun 27, 2022 | 25 (current) | download |
| Mar 7, 2022 | 24 | download |
| Sep 21, 2021 | 22 | download |
| Dec 31, 2020 | 21 | download |
| Aug 18, 2020 | 20 | download |
| Sep 25, 2019 | 19 | download |
| Aug 6, 2018 | 18 | download |
| Nov 22, 2017 | 17 | download |
| Nov 28, 2016 | 16 | download |
| Jan 28, 2016 | 15 | download |
| Jun 15, 2015 | 14 | download |
| Jan 30, 2015 | 13 | download |
| Nov 25, 2013 | 12 | download |
| Nov 16, 2012 | 11 | download |
| Jun 14, 2012 | 10 | download |
| Jan 3, 2012 | 9 | download |
| Nov 17, 2011 | 8 | download |
| Jul 7, 2011 | 7 | download |
| Oct 18, 2010 | 6 | download |
| Sep 20, 2010 | 5 | download |
| Aug 17, 2010 | 4 | download |
| Aug 13, 2010 | 3 | download |
| Jul 20, 2010 | 2 | download |
| Nov 12, 2009 | 1 | download |
RxNorm
LANSOPRAZOLE capsule, delayed release
Get Label RSS Feed for this Drug
LANSOPRAZOLE capsule, delayed release
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=427cf924-be17-47dc-8f77-115fa2f3fe17
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LANSOPRAZOLE capsule, delayed release
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0093-7350-56 |
| 2 | 0093-7351-56 |


