Label: TOLTERODINE TARTRATE capsule, extended release
- NDC Code(s): 0093-7163-05, 0093-7163-56, 0093-7163-98, 0093-7164-05, view more
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TOLTERODINE TARTRATE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for TOLTERODINE TARTRATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETolterodine tartrate extended-release capsules are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency [see CLINICAL STUDIES ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended dose of tolterodine tartrate extended-release capsules is 4 mg once daily with water and swallowed whole. The dose may be lowered to 2 mg daily based on ...
-
3 DOSAGE FORMS AND STRENGTHSThe 2 mg capsules are light green with “7163” imprinted on the body and “TEVA” imprinted on the cap. The 4 mg capsules are aqua blue with “7164” imprinted on the body and “TEVA” imprinted on the ...
-
4 CONTRAINDICATIONSTolterodine tartrate extended-release capsules are contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. Tolterodine tartrate ...
-
5 WARNINGS AND PRECAUTIONS5.1 Angioedema - Anaphylaxis and angioedema requiring hospitalization and emergency medical treatment have occurred with the first or subsequent doses of tolterodine tartrate extended-release ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
7 DRUG INTERACTIONS7.1 Potent CYP2D6 Inhibitors - Fluoxetine, a potent inhibitor of CYP2D6 activity, significantly inhibited the metabolism of tolterodine immediate release in CYP2D6 extensive metabolizers ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with tolterodine tartrate extended-release capsules use in pregnant women to inform drug-associated risks. In animal reproduction ...
-
10 OVERDOSAGEOverdosage with tolterodine tartrate extended-release capsules can potentially result in severe central anticholinergic effects and should be treated accordingly. ECG monitoring is recommended in ...
-
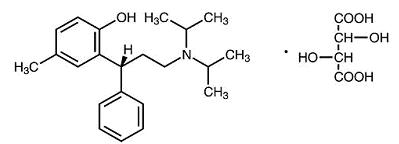
11 DESCRIPTIONTolterodine tartrate extended-release capsules contain Tolterodine Tartrate, USP. The active moiety, tolterodine, is a muscarinic receptor antagonist. The chemical name of Tolterodine Tartrate ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tolterodine acts as a competitive antagonist of acetylcholine at postganglionic muscarinic receptors. Both urinary bladder contraction and salivation are mediated via ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies with tolterodine were conducted in mice and rats. At the maximum tolerated dose in mice (30 mg/kg/day), female ...
-
14 CLINICAL STUDIESTolterodine tartrate extended-release capsules, 2 mg were evaluated in 29 patients in a Phase 2 dose-effect study. Tolterodine tartrate extended-release capsules, 4 mg were evaluated for the ...
-
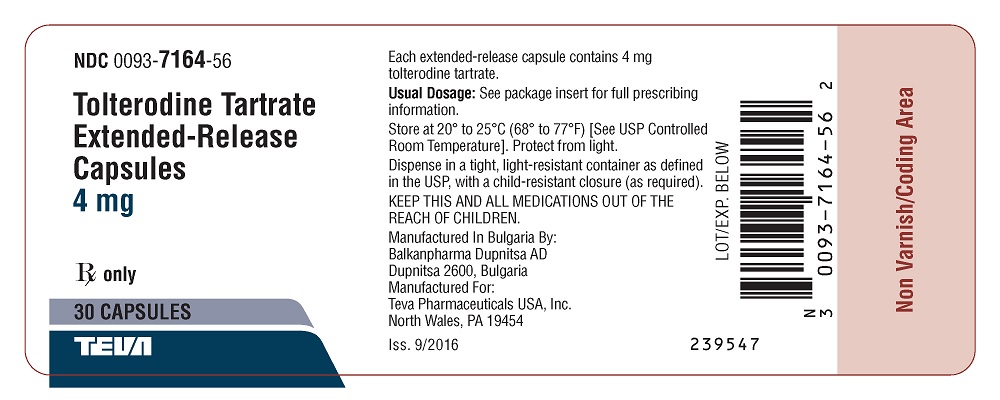
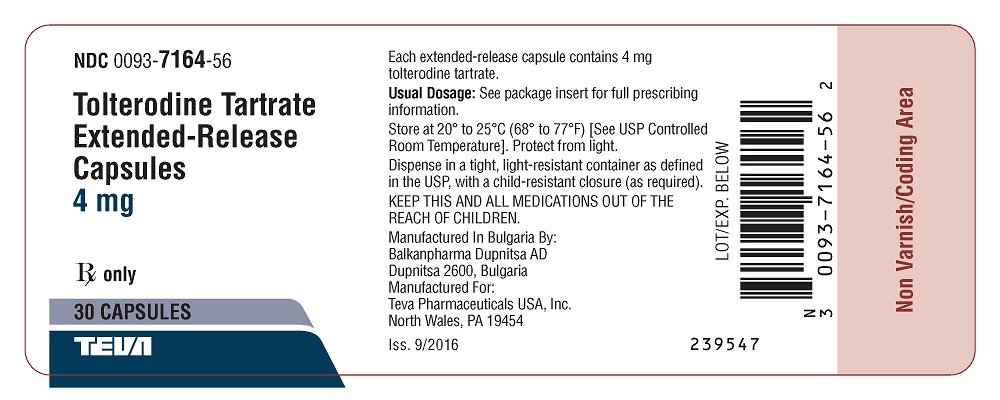
16 HOW SUPPLIED/STORAGE AND HANDLINGTolterodine tartrate extended-release capsules are available as follows: 2 mg: A hard gelatin capsule with a light green opaque cap and body, filled with white to off-white pellets, imprinted on ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Antimuscarinic Effects - Inform patients that antimuscarinic agents such as tolterodine tartrate ...
-
PATIENT PACKAGE INSERTDispense with Patient Package Insert available at: www.tevausa.com/PatientPI - PATIENT INFORMATION - Tolterodine (tol terˈ oh deen tarˈ trate) Tartrate Extended-Release Capsules - Read the Patient ...
-
Package/Label Display PanelNDC 0093-7163-56 - Tolterodine Tartrate - Extended-Release - Capsules - 2 mg - Rx only - 30 Capsules
-
Package/Label Display PanelNDC 0093-7164-56 - Tolterodine Tartrate - Extended-Release - Capsules - 4 mg - Rx only - 30 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information